
Filed Pursuant to Rule 424(b)(3)
Registration No. 333-280000
UP TO 3,500,000
AMERICAN DEPOSITARY SHARES
REPRESENTING 70,000,000 ORDINARY SHARES
TC BIOPHARM (HOLDINGS) PLC

This prospectus relates to the offer and resale of series F ordinary share purchase warrants (the “Warrants”) to purchase up to 3,500,000 American Depositary Shares (the “ADSs”), which were issued by us pursuant to a letter agreement dated May 6, 2024 (the “Letter Agreement”). Each ADS represents twenty (20) of our ordinary shares, par value £0.0001 per share. The holders of the ADSs are each referred to herein as a “Selling Shareholder”.
The exercise price of the Warrants is £1.175 per ADS ($1.469 per ADS translated for illustration to U.S. dollars at the rate of £1.00 to $1.2503 as of May 8, 2024). See “Use of Proceeds”. The Selling Shareholder, or its transferees, pledgees, donees or other successors-in-interest, may sell the ADSs through public or private transactions at prevailing market prices, at prices related to prevailing market prices or at privately negotiated prices. The Selling Shareholder may sell any, all or none of the securities offered by this prospectus, and we do not know when or in what amount the Selling Shareholder may sell their ADSs hereunder following the effective date of this registration statement. We provide more information about how a Selling Shareholder may sell its ADSs in the section titled “Plan of Distribution” on page 41.
We are registering the ADSs on behalf of the Selling Shareholder to be offered and sold by them from time to time. While we will not receive any proceeds from the sale of the ADSs by any selling shareholder, we will receive proceeds from the exercise of any Warrants for cash. We have agreed to bear all of the expenses incurred in connection with the registration of the ADSs. The Selling Shareholder will pay or assume discounts, commissions, fees of underwriters, selling brokers or dealer managers and similar expenses, if any, incurred for the sale of the ADSs.
Our ADSs are listed on the Nasdaq Capital Market, or Nasdaq, under the symbol “TCBP”. On June 18, 2024, the closing trading price for our ADSs, as reported on Nasdaq, was US$0.9498 per ADS.
We an “emerging growth company” each as defined under the federal securities laws, and, as such, we are subject to reduced public company reporting requirements. See the section entitled “Prospectus Summary—Implications of Being an Emerging Growth Company” for additional information.
Investing in our securities involves a high degree of risk. Before buying any ADSs, you should carefully read the discussion of material risks of investing in the ADSs and the company. See “Risk Factor Summary” beginning on page 28 for a discussion of information that should be considered in connection with an investment in our securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Prospectus dated June 24, 2024
TABLE OF CONTENTS
We have not authorized anyone to provide information different from that contained in this prospectus, any amendment or supplement to this prospectus or in any free writing prospectus prepared by us or on our behalf. We take no responsibility for, and can provide no assurance as to the reliability of, any information other than the information in this prospectus, any amendment or supplement to this prospectus, and any free writing prospectus prepared by us or on our behalf. Neither the delivery of this prospectus nor the sale of the ADSs means that information contained in this prospectus is correct after the date of this prospectus. This prospectus is not an offer to sell or the solicitation of an offer to buy the ADSs in any circumstances under which such offer or solicitation is unlawful.
You should rely only on the information contained in this prospectus and any free writing prospectus prepared by or on behalf of us or to which we have referred you. We have not authorized anyone to provide you with information that is different. We are offering to sell the ADSs, and seeking offers to buy the ADSs, only in jurisdictions where offers and sales are permitted. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of the ADSs.
For investors outside of the United States, we have not done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions relating to this offering and the distribution of this prospectus outside the United States.
| -2- |
This prospectus is part of a registration statement on Form S-3 that we filed with the United States Securities and Exchange Commission (the “SEC”) using a “shelf” registration process. Under this shelf registration process, the selling securityholders may, from time to time, offer and sell any combination of the securities described in this prospectus in one or more offerings.
This prospectus provides you with a general description of the securities we and the Selling Shareholders may offer. This prospectus and any accompanying prospectus supplement do not contain all of the information included in the registration statement. We have omitted parts of the registration statement in accordance with the rules and regulations of the SEC. Statements contained in this prospectus and any accompanying prospectus supplement about the provisions or contents of any agreement or other documents are not necessarily complete. If the SEC rules and regulations require that an agreement or other document be filed as an exhibit to the registration statement, please see that agreement or document for a complete description of these matters. This prospectus may be supplemented by a prospectus supplement that may add, update, or change information contained or incorporated by reference in this prospectus. You should read both this prospectus and any prospectus supplement or other offering materials together with additional information described under the headings “Where You Can Find Additional Information” and “Incorporation of Documents by Reference.”
Each time we sell any securities offered by us under this shelf registration, we will provide a prospectus supplement that will contain certain specific information about the terms of that offering, including a description of any risks related to the offering. A prospectus supplement may also add, update, or change information contained in this prospectus (including documents incorporated herein by reference). Notwithstanding the foregoing, the Selling Shareholders may sell the ADSs offered by them registered hereby without being accompanied by a prospectus supplement. If there is any inconsistency between the information in this prospectus and the applicable prospectus supplement, you should rely on the information in the prospectus supplement. The registration statement we filed with the SEC includes exhibits that provide more details on the matters discussed in this prospectus. You should read this prospectus and the related exhibits filed with the SEC and the accompanying prospectus supplement together with additional information described under the headings “Incorporation of Documents by Reference” before investing in any of the securities offered.
You should rely only on the information provided or incorporated by reference in this prospectus or in the prospectus supplement. Neither we nor the Selling Shareholders have authorized anyone to provide you with additional or different information. Neither we nor the Selling Shareholders take responsibility for, nor can we provide assurance as to the reliability of, any other information that others may provide. Neither we nor the Selling Shareholders are making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information in this prospectus, any applicable prospectus supplement or any related free writing prospectus is accurate only as of the date on the front of the document and that any information incorporated by reference is accurate only as of the date of the document incorporated by reference, regardless of the time of delivery of this prospectus, any applicable prospectus supplement or any related free writing prospectus, or any sale of a security, unless we indicate otherwise. Our business, financial condition, results of operations and/or prospects may have changed since those dates.
As permitted by SEC rules and regulations, the registration statement of which this prospectus forms a part includes additional information not contained in this prospectus. You may read the registration statement and the other reports we file with the SEC at its website or at its offices described below under “Where You Can Find Additional Information.”
Unless the context requires otherwise, in this prospectus TC BioPharm (Holdings) plc (formerly TC BioPharm (Holdings) Limited, which was re-registered as a public limited company on January 10, 2022) and its subsidiaries (“Subsidiar(y/ies)”), and TC BioPharm Limited (our principal trading subsidiary) shall collectively be referred to as “TCB,” “the Company,” “the Group”, “we,” “us,” and “our” unless otherwise noted.
| -3- |
On December 17, 2021, prior to our initial public offering, the Company undertook a corporate reorganization pursuant to which TC BioPharm (Holdings) plc became the group holding company. The Company in turn effected a forward split of its ordinary shares on a 10 for 1 basis. On November 18, 2022 the Company undertook a reverse share split such that fifty issued ordinary share were exchanged for one new ordinary share. As a result of the share splits, all references included in this document to units of ordinary shares or per share amounts are reflective of the forward and reverse share splits for all periods presented. In addition, the exercise prices and the numbers of ordinary shares issuable upon the exercise of any outstanding options to purchase ordinary shares were proportionally adjusted pursuant to the respective anti-dilution terms of the share-based payment plans.
The consolidated financial statement data as at December 31, 2023 and 2022, and for the years ended December 31, 2023 and 2022 have been derived from our consolidated financial statements, which have been prepared in accordance with generally accepted accounting principles, or GAAP. The December 31, 2023 and 2022 consolidated financial statements were audited in accordance with the standards of the Public Company Accounting Oversight Board (United States).
This prospectus includes statistical, market and industry data and forecasts which we obtained from publicly available information and independent industry publications and reports that we believe to be reliable sources. These publicly available industry publications and reports generally state that they obtain their information from sources that they believe to be reliable, but they do not guarantee the accuracy or completeness of the information. Although we believe that these sources are reliable, we have not independently verified the information contained in such publications. In addition, assumptions and estimates of our and our industry’s future performance are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in the “Risk Factor Summary”. These and other factors could cause our future performance to differ materially from our assumptions and estimates.
Some of our trademarks and trade names are used in this prospectus, which are intellectual property owned by the Company. This prospectus also includes trademarks, trade names, and service marks that are the property of other organizations. Solely for convenience, our trademarks and trade names referred to in this prospectus appear without the TM symbol, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or the right of the applicable licensor to these trademarks and trade names.
No offer of these securities will be made in any jurisdiction where the offer is not permitted.
ENFORCEABILITY OF CIVIL LIABILITIES
TCB is a corporation organized under the laws of Scotland. Substantially all of TCB’s assets and the majority of its directors and executive officers are located and reside, respectively, outside the United States. Because of the location of TCB’s assets and board members, it may not be possible for investors to serve process within the United States upon TCB or those persons with respect to matters arising under the United States federal securities laws or to enforce against TCB or persons located outside the United States judgments of United States courts asserted under the civil liability provisions of the United States federal securities laws.
TCB understands that there is doubt as to the enforceability in Scotland and the United Kingdom, in original actions or in actions for enforcement of judgments of United States courts, of civil liabilities predicated solely upon the federal securities laws of the United States insofar as they are fines or penalties. In addition, awards of punitive damages in actions brought in the United States or elsewhere may be unenforceable in Scotland and the United Kingdom by reason of being a penalty.
TC BioPharm (North America) Inc., a Delaware corporation, with a registered office at Business Filings, Inc. 108 West 13th Street, Wilmington, Delaware 19801, has been appointed agent to receive service of process in any action against TC BioPharm (Holdings) plc in any state or federal court in the State of New York.
| -4- |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
TCB discusses in this prospectus its business strategy, market opportunity, capital requirements, product introductions and development plans and the adequacy of the Company’s funding. Other statements contained in this prospectus, which are not historical facts, are also forward-looking statements. TCB has tried, wherever possible, to identify forward-looking statements by terminology such as “may,” “will,” “could,” “should,” “expects,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “estimates” and other comparable terminology.
TCB cautions investors that any forward-looking statements presented in this prospectus, or that TCB may make orally or in writing from time to time, are based on the beliefs of, assumptions made by, and information currently available to, TCB. These statements are based on assumptions, and the actual outcome will be affected by known and unknown risks, trends, uncertainties and factors that are beyond its control or ability to predict. Although TCB believes that its assumptions are reasonable, they are not a guarantee of future performance, and some will inevitably prove to be incorrect. As a result, its actual future results can be expected to differ from its expectations, and those differences may be material. Accordingly, investors should use caution in relying on forward-looking statements, which are based only on known results and trends at the time they are made, to anticipate future results or trends. Certain risks are discussed in this prospectus and also from time to time in TCB’s other filings with the Securities and Exchange Commission (“SEC”).
This prospectus and all subsequent written and oral forward-looking statements attributable to the Company or any person acting on its behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section. The Company does not undertake any obligation to release publicly any revisions to its forward-looking statements to reflect events or circumstances after the date of this prospectus.
In particular, you should consider the risks provided under “Risk factors” in this prospectus and in the Form 10-K for the fiscal year ended December 31, 2023 as filed with the Securities and Exchange Commission (the “2023 Form 10-K”) incorporated by reference in this prospectus.
| -5- |
The following summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all the information you should consider before investing in our securities. You should read this entire prospectus carefully, including the information incorporated by reference in this prospectus and any free writing prospectus prepared by us or on our behalf, including in particular the section entitled “Risk Factors” in this prospectus,
The Company
Corporate Overview
TCB based in Scotland, is a clinical-stage biopharmaceutical company focused on developing novel immunotherapy products based on our proprietary allogeneic gamma delta T (GD-T) cell platform. Harnessing the innate ability of GD-Ts has enabled us to develop a range of clinical-stage cell therapies designed to combat cancer and viral infection.
In-house clinical studies have demonstrated that our unmodified allogeneic GD-T products are (i) well tolerated and (ii) show preliminary evidence of disease modification in patients with the late-stage blood cancer, known as acute myeloid leukemia (AML). Based on clinical data generated by us believe that unmodified GD-Ts have the potential to treat all blood cancers.
TCB now is embarking on phase 2b-into-pivotal (phase 3) clinical studies with a view to launching its first oncology product for the treatment of AML. Clinical results generated thus far have enabled us to obtain FDA orphan drug status for treatment of AML.
In addition to unmodified allogenic GD-Ts for treatment of blood cancers, we are also developing an innovative range of genetically-modified CAR-T products for treatment of solid cancers. We believe that solid cancers are more difficult to treat than blood cancers and may require the addition of a CAR “chimeric antigen receptor” (i) to help therapeutic cells to “navigate” into diseased cancerous tissue and (ii) to retain therapeutic cells in-situ at the lesion for maximal efficacy (increased persistence).
In order to manufacture our portfolio of allogeneic products, TCB selects the highest quality GD-T cells from healthy donors, activate the cells and grow them in large numbers at our in-house GMP-compliant manufacturing facility before administration to a patient in order to target and then destroy malignant or virally-infected tissues. TCB believes that we have introduced a step-change to our manufacturing platform by implementing a freeze-thaw process that will allow product to be shipped from cleanroom to patient without any shelf-life issue. Resulting products, TCB believes, will be more cost-effective and straightforward to ship form cleanroom to clinic.
At this stage, TCB does not have any approved products. Accordingly, TCB has not generated any revenue from the sale of products, and TCB does not expect to generate any such revenue unless and until it obtains regulatory approvals for, and commercialize any of, our product candidates. In the future, TCB will seek to generate revenue primarily from product sales and, potentially, regional or global collaborations with strategic partners, which may produce license fee income.
See “Business - Overview” in 2023 Form 10-K incorporated by reference in this prospectus.
Intellectual Property
We have a strong portfolio of patents covering manufacture and commercialization of GD-T cell products and their modification via CAR-T (summarized below). Our technology platform and clinical programs have enabled us to raise over $100 million in grant, equity and collaboration funding since becoming operational in 2017. This financing has allowed us to enhance and expand our clinical and preclinical programs as well as build our team of world-class scientists.
| -6- |
The following table provides an overview of our core technology platforms, technology assets and competencies across the business. Additional details of our intellectual property portfolio are provided below.
| ASSET SUMMARY | ATTRIBUTES | ||
| GD-T Vehicle | ● | Readily available and expanded to high numbers. | |
| ● | Not MHC-restricted, therefore no graft vs host disease – an allogeneic platform. | ||
| ● | Pre-programmed tropism for infiltration of diseased tissue. | ||
| ● | Multiple modes of innate cytotoxicity and coordinating a wider immune response. | ||
| ● | Clinical tolerability of the allogeneic vehicle demonstrated at high dose level. | ||
| ● | Naturally arising in different subtypes offering a menu of vehicles with unique properties. | ||
| Allogeneic Cell Banks | ● | Donor GD-Ts selection based on highest therapeutic quality. | |
| ● | Reproducible product with low cost-of-goods compared with autologous (patient-bespoke) therapies, can be frozen-shipped, thawed at clinic. | ||
| ● | Well understood clinical and regulatory pathway to commercialization. | ||
| Co-stimulatory CAR-T | ● | Elimination of off-tumor toxicity. | |
| ● | Reduction of cytokine release from killing healthy cells. | ||
| ● | Reliance on natural T cell activation and no tonic signaling | ||
| ● | Antigen expression on healthy tissue tolerated – greatly expanded range. | ||
| ● | Ability to use multiple co-stimulatory receptors to add functionality. | ||
| Integrated Business Model | ● | Full control of critical stages of development projects, which increases speed and reliability of development and production, optimizes operations to our specialized products and materially reduces our cost base | |
| ● | No pass-through or transaction costs form external service providers, which increases efficiency and speed of development and manufacturing and materially reduces our cost base | ||
| ● | In-house clinical management ensures best chance of clinical success and avoids use of very expensive clinical management in early-stage trials, materially reducing our cost base. |
The strength of our patents involves complex legal and scientific questions and can be uncertain.
We actively seek to protect the intellectual property and proprietary technology that we believe is important to our business, including seeking, maintaining, enforcing and defending patent rights for our therapeutics and processes, whether developed internally or licensed from third parties. Our success will depend on our ability to obtain and maintain patent and other protection including data/market exclusivity for our therapeutic products and platform technology, preserve the confidentiality of our know-how and operate without infringing the valid and enforceable patents and proprietary rights of third parties.
Our policy is to seek to protect our proprietary position generally by filing an initial priority filing at the U.K. Intellectual Property Office, or UKIPO. This is followed by the filing of a patent application under the Patent Co-operation Treaty claiming priority from the initial application(s) and then progressing to national applications in, for example, the United States, Europe, Japan, Australia, New Zealand, China and Canada. In each case, we determine the strategy and territories required after discussion with our patent professionals to ensure that we obtain relevant coverage in territories that are commercially important to us and our GD-T therapeutic candidates. We will additionally rely on data exclusivity, market exclusivity and patent term extensions when available, including as relevant exclusivity through orphan or pediatric drug designations. We also rely on trade secrets and know-how relating to our underlying platform technology and therapeutic products. Prior to making any decision on filing any patent application, we consider, with our patent professionals, whether patent protection is the most sensible strategy for protecting the invention concerned or whether the invention should be maintained as confidential.
| -7- |
As of June 19, 2024, we own 16 granted patents and 11 patent applications in 3 families, and have an exclusive license to an additional 1 family of 14 granted patents and 8 patent applications. Consistent with the filing strategy outlined above, all of our applications are either UK applications, PCT applications or national phase applications derived from a corresponding PCT application. These patents and patent applications include claims directed to our therapeutic products and platform technology or other manufacturing and process technology to further enable our therapeutic products and manufacturing methods.
See “Business - Intellectual Property” in our 2023 Form 10-K incorporated by reference in this prospectus.
Our Business Strategy
We have taken a step-wise approach to clinical development and commercialization. To achieve this, we have made the clinical transition from autologous GD-Ts to allogeneic GD-Ts, improving our process for optimization of our product based on data and new technologies. The Company plans to maximize the value of TCB-008 and future iterations by expanding the use case for the product, effectively believing TCB-008 (and future such iterations) to be a “platform therapeutic” based upon it’s safety profile and the in-house knowledge of GD-Ts and TCB-008. Additionally, the Company plans to opportunistically add to the asset base of the Company around other cell therapy approaches and such technologies where we can leverage our expertise and facilities. . Our commercialization strategy is to introduce products firstly in blood cancers (AML initially), and pending data, in other disease indications and in solid tumors as a combination therapeutic..
Our strategic objective is to build a global therapeutic business with an extensive portfolio of GD-T cell-based products with the potential to significantly improve the outcomes of patients with cancer and infectious disease. In order to achieve our objective, we are focused on delivering success in the following areas:
Progress unmodified GD-T2s into Phase 2/3 clinical trials for the treatment of blood cancers
Having generated meaningful clinical data showing our product is well-tolerated in late-stage AML patients with no remaining treatment options, we commenced phase 2b-into pivotal (phase 3) clinical studies under the trial name ‘ACHIEVE’, with OmnImmune® during 2022 in AML patients who have failed to respond adequately to induction therapy. The aim is to provide a form of salvage therapy which will either stabilize the patient, thereby preventing disease progression, or delay the requirement for human stem cell transplant. Our initial trial centers are in the UK and we are currently dosing patients in this trial. Working on the premise that other blood cancers should respond to GD-Ts in a similar manner to AML, TCB plans to conduct clinical studies for OmnImmune® in other hematological malignancies in future.
Unmodified GD-T2s for use in the treatment of fungal infections
Gamma-delta T cells are dysfunctional in patients with many severe viral diseases and TCB anticipates that its unmodified gamma delta T cell therapy platform will be used in due course to treat viral infections as well as cancers under the name ImmuniStim®. For example, during 2022 TCB developed a clinical trial protocol to treat patients with COVID 19. Because of the progress of the disease and absence of appropriate trial patients this trial is not currently being progressed, although we expect to continue our infectious disease program in future.
| -8- |
Grow our business operations to support the increasing number of clinical-phase products in development
We believe that our existing cell and gene manufacturing facility in the UK has the capacity to support our committed clinical development plans. We plan to continue to build upon this to support expansion of our product pipelines to new assets and to grow our clinical team. We also will work closely with vendors to embrace emerging technologies in our manufacturing operations that are appropriate and optimized for our products to continually improve the quality and efficiency of our manufacturing systems. We believe that maintaining in-house control of these activities is critical to effective and efficient progression and we will continue to seek to build integrated business functions where possible.
Apply our discovery engine to target further diseases and add additional functionality to our products
As a platform technology, our co-stimulatory CAR-T GD-T cell system has a wealth of potential options to build added functionality into our cell-based platform. We plan to continue to innovate and partner in the field to augment our drug products and introduce next generation attributes. We also plan to continue to innovate our manufacturing and supply chains to efficiently scale our processes and simplify the interface with patients and healthcare professionals, whilst continually seeking to reduce manufacturing costs to improve patient access.
Expand our intellectual property portfolio and acquire additional technologies to augment our strong IP position
We intend to continue building on our technology platform, comprised of intellectual property, proprietary methods and know-how in the field of GD-T cells. These assets form the foundation for our ability, not only to strengthen our product pipeline, but also to successfully defend and expand our position as a leader in the field of GD-T based immune-oncology.
See “Business - Business Strategy” in our 2023 Form 10-K incorporated by reference in this prospectus.
TCB’s Strengths
Our clinical trials have provided very strong evidence of drug-toleration and some preliminary evidence of clinical benefit.
Our clinical trial of TCB001 involved treatment of patients with autologous unmodified GD-Ts. In a phase 1b/2a dose-ranging safety study (maximum total dose 30x109 cells) we saw no evidence of drug-related severe adverse events. A total of eight patients were treated with escalating doses of TCB001, and no treatment-related toxicities were reported during the full six-week therapeutic course. Data from OmnImmune® (TCB002) suggests an excellent tolerability, with no observed Host versus Graft Disease (HvGD) and some preliminary indication of clinical benefit. OmnImmune® (TCB002) has been granted Orphan Drug Designation by the FDA.
Our CAR-T platform is centered on development of safer and more widely applicable therapeutic candidates and associated process and manufacturing capabilities.
Our proprietary co-stimulatory CAR-T technology platform covers identification of target cancer antigens, successful design and engineering of target sequences, preclinical safety testing and optimized manufacturing processes suitable for producing therapeutic candidates for use in clinical trials and commercialization. We believe the platform will enable development of additional GD-T cell therapeutic candidates targeting cancers that have previously been difficult to treat. We believe the products will be demonstrably safer than the current generation of AB T cell CAR-T products because they will not attack healthy non-cancerous cells and augment the natural biological process rather than bypassing it.
We have identified a large and growing pool of cancer targets for which we can develop additional therapeutic candidates.
We have identified over 20 antigens that are preferentially expressed in cancer cells and have established ongoing research programs to develop several of these into our GD-T platform. Within the terms of our agreement, bluebird bio, we have first right of refusal on a further three oncology targets. Each antigen target presents an opportunity to target many cancer types and therefore presents multiple potential represents a development, collaboration and/or an out-licensing opportunity as each target could be used to target specific cancer types. Growing the pipeline of products built on our co-stimulatory CAR-T and reaching patients is our priority.
| -9- |
We have historically entered collaborative arrangements with partners (bluebird bio, Inc (now 2seventybio). (USA) and Nipro Corporation (Japan), which involve funded or partly funded preclinical collaboration. It is uncertain at this time whether TCB will receive any significant revenues from these collaborations.
We retain control of key business elements, such as product manufacture and clinical research.
Whilst many companies contract out product manufacture, quality systems and clinical trial management, we have elected to build these skills in-house. TC BioPharm has a GMP (Good Manufacturing Practice) cleanroom facility where our products are manufactured. We also retain all the quality support systems such as product testing and release of final product to the clinic. Keeping these systems in-house allows the Company to control all aspects of the manufacturing process whilst significantly reducing costs of goods (CoGs). Further saving on costs are accrued by in-house manufacture, as contract manufacturing organizations (CMOs) will typically charge several times more than the actual costs to maintain their profit margins. Rather than fully outsource our clinical trial management, data management and pharmacovigilance, we maintain an inhouse clinical team that partners with a contract clinical research organization (CRO) for data management and pharmacovigilance services. The inhouse clinical team conducts and manages our own clinical trials in-house. In addition to significant cost savings, this allows us to build a strong working relationship with physicians who are treating the cancer patients; we believe this is key to successful product development as the physicians participating in our clinical studies will also be our future customers. We believe that retaining control of key elements of our business such as GMP manufacture and clinical operations, has allowed TC BioPharm to move quickly and efficiently since incorporation.
We continue to file new patent applications from new in-house product development, and have a strong growing intellectual property portfolio to protect our products and proprietary platform.
We have a strong intellectual property portfolio covering the key aspects of our manufacturing processes and product platforms. Our in-house product development team are dedicated to developing new therapeutic candidates and optimizing current manufacturing processes. All of our patent families are currently in various stages of the patent approval process, and as leaders in the path towards the commercialization of GD-Ts we hold significant first-mover advantage captured by trade secrets and know-how.
Our policy of developing strategic alliances has and will provide additional support for product development and commercialization.
We believe that strategic alliances, both historic and potential future alliances, have and will provide extensive experience in scale-up and automation, culture media manufacture and post-authorization sales and marketing with regional expertise. Additionally, we expect to use knowledge gained from our collaborations to improve development pathways for our unpartnered CAR-T therapeutic candidate programs.
We have a highly knowledgeable and experienced management team with extensive industry experience and expertise in the United States and in Europe.
Mr. Kobel joined us as our Chief Executive Officer at the time of our IPO. Bryan brings a US presence to our executive team and over 15 years’ experience in Healthcare and Life Sciences capital markets. Martin Thorp, our Chief Financial Officer has over 40 years’ experience in implementing capital strategies globally from seed investment to IPO. He was global CEO of Arthur Andersen Corporate Finance based in New York.
Ability to treat patients under the ‘Specials’ regulatory framework.
European regulations (Regulation 167 of the Human Medicines Regulations 2012) set out the exemption from the requirement for a medicinal product, placed on the market in the UK to hold a marketing authorization. This exemption flows from Article 5(1) of EU Directive 2001/83/EC, which states that a member of the EU may, in accordance with legislation in force and to fulfil special needs, excludes from the provisions of this Directive medicinal products supplied in response to a bona fide unsolicited order, formulated in accordance with the specifications of an authorized healthcare professional and for use by an individual patient under his or her direct personal responsibility. Such an unlicensed medicinal product may only be supplied in order to meet the special needs of an individual patient. An unlicensed medicinal product should not be supplied where an equivalent licensed medicinal product can meet the special needs of the patient. Responsibility for deciding whether an individual patient has “special needs” which a licensed product cannot meet should be a matter for the doctor, dentist, nurse independent prescriber, pharmacist independent prescriber or supplementary prescriber responsible for the patient’s care.
| -10- |
In terms of time and cost, the ‘Specials’ scheme is an attractive strategy. We believe that accumulating evidence by this route could lead to rapid and wider product uptake through ‘off-label’ use.
TCB believes it has certain identified strengths. These include:
| ● | Clinical trials that have provided strong evidence of safety and some preliminary indications of clinical benefit; | |
| ● | A proprietary co-stimulatory CAR-T technology platform which we believe allows solid cancers to be treated without toxic side-effects; | |
| ● | Identification of a large pool of cancer targets for which we believe we can develop therapeutic candidates; | |
| ● | Retention of key business elements, especially in-house ability to manufacture cell-based product and conduct our own clinical research; | |
| ● | Robust, and growing intellectual property portfolio protecting our products and proprietary platform; | |
| ● | Our policy is to develop strategic collaborations with leading, international companies to work together with us to develop certain GD-T CAR-T products into clinic. We believe that existing and future collaborations will provide us with experience in scale-up and automation, and post-authorization sales and marketing; | |
| ● | A highly knowledgeable and experienced management team with extensive industry experience and expertise in the United States and in Europe; and | |
| ● | Ability to treat patients under the ‘Specials’ regulatory framework in Europe. |
Our Pipeline
What are gamma delta T cells?
The immune system plays an important role in targeting and destroying cancer cells. One component has evolved to scan the body for diseased cells and eradicate them. In humans, GD-Ts arise as a number of different subtypes, defined by the sequence of the gamma and delta chains of the T-cell receptor (TCR) on the cell surface. The gammadelta2 (GD-T2) subtype typically is the most abundant of these cells in healthy humans, and its TCR- of anti-cancer immunity is GD-T cells – a type of white blood cell that express a variety of innate receptors, which mediated signaling has been fully characterized by researchers.
Virally-infected or cancerous cells become stressed and accumulate cell surface phosphoantigens (isopentenyl pyrophosphate – IPP’s) which are recognized by GD-T2 cells. Our proprietary technology platform includes the manufacturing of unmodified and genetically modified (CAR-T) GD-T cells as therapeutic candidates for use in clinical trials and commercialization. Almost all aspects of the value-chain from product manufacture, quality systems, clinical and regulatory are operated in-house by TC BioPharm. We believe this is one of our core competitive advantages, which we believe will contribute materially to our ability to overcome the challenging nature of developing new products.
Human lymphocytes comprise two groups of cells, B cells that generate antibodies for humoral immunity, and T cells that are responsible for cellular immune responses. In healthy individuals, GD-T cells generally represent between 1% and 10% of peripheral blood T lymphocytes and present one of the first lines of defense against a wide range of bacterial and viral pathogens, as well as surveillance for cancerous cells. GD-T cells have the ability to regulate the initial immune response in several ways, including recruitment of other immune cells such as neutrophils, dendritic cells and macrophages through production of various chemokines (Kirby et al., 2007). Depletion of GD-T cells leads to impaired host defense to lung infections, for example (Moore et al., 2000; Lockhart et al., 2006). The predominant subset of GD-T cells in the blood is the GD-T2, which mediates a variety of immune responses by direct cytolysis of cancer cells and infected cells, development of memory phenotypes and modulation of other immune cells. The gammadelta1 (GD-T1) is a functionally distinct subset of GD-T cells, which are a predominantly tissue resident population. GD-T1s are less well characterized, but their cytotoxic function also has been described in different liquid and solid tumors (Siegers & Lamb, 2014).
| -11- |
Both subsets of GD-T cells are thought to play a role in autoimmune disorders such as celiac disease, rheumatoid arthritis, autoimmune polyglandular syndrome and sarcoidosis where such lymphocytes are seen to accumulate in high numbers.
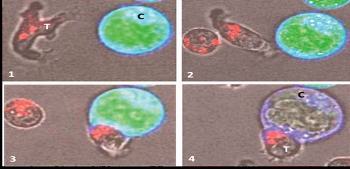 |
GD-T cell killing a cancer cell.
(1) A human GD-T (labelled ‘T’) identifies and scans (2) the surface of a cancer cell (labelled ‘C’). On contact with the cancer cell (3) the GD-T releases perforin granules (stained red) into the cancer cell, rupturing its membrane (4) destroying the cancer cell (adapted from – Enc Life Sci, Jul-2007). |
How can GD-Ts be used to treat disease?
Cellular immunotherapy is a form of treatment that harnesses the cells of the immune system to combat disease and is one of the most actively pursued areas of research by biotechnology and pharmaceutical companies today. Interest in immunotherapy is largely driven by recent compelling efficacy data in cancers and by the potential to achieve a cure or functional cure for some patients. While the field of immunotherapy in cancer, in general, has achieved proof of concept and yielded significant durable responses in multiple tumor types, there remain major tumor types such as colon, breast, and prostate cancers as well as patient groups within responsive tumors, that do not respond to current immunotherapy treatments. One theory to explain this non-responsiveness is that certain tumors require direct immune stimulation. T cell-based technologies seek to deliver activated T cells towards malignancies to initiate an immune response. The primary challenges in the field have been to couple an acceptable efficacy and safety profile to successfully target solid tumors.
Adoptive T cell transfer typically involves administration of autologous, allogeneic, or genetically-modified T cells (see footer below) into a recipient host with the specific goal of boosting or transferring enhanced immunologic functionality. One of the most advanced cell-based approaches – chimeric antigen receptor modified T cells (CAR-T) – has gained momentum. In a recent study, patients with refractory B cell acute lymphoblastic leukemia were treated with autologous genetically-modified T cells, with almost 90% of patients showing a marked improvement (Pan et al., 2017). Although the treatment is showing promise for specific tumor types, the safety profile remains a concern, as serious adverse events have previously been reported following CAR-T therapy (Grigor et al., 2017). As a consequence of safety issues related to this approach, regulatory approval may be more complex for this genetically modified T cell therapy which effectively has two ‘starting materials’ – (i) the cellular component, and (ii) a lentiviral vector. The therapeutic premise is well-established – T cells are transduced with a viral vector encoding a chimeric antigen receptor capable of recognizing cancer-specific antigens, for example, CD19 which is commonly expressed on several tumors such as myeloma and B cell lymphomas. Transduction is the process by which DNA is transferred from one cell to another by a virus; in this specific case DNA is introduced via a viral vector (a tool commonly used by molecular biologists to deliver genetic material).
Following transduction, the T cells are genetically primed to recognize and kill specific tumor cells expressing the target antigen. The process involves extracting a patient’s T cells (or growing an allogeneic T cell bank), transfecting the cells with a gene for a chimeric-antigen-receptor (CAR), and re-infusing transfected T cells into the patients. The use of cancer-specific cell therapies has gained momentum as several companies demonstrated that genetically modified CAR-T cells are efficacious when directed against blood tumors. These breakthrough findings have moved cell-based immunotherapy into the forefront of clinical oncology with two drugs now in the market.
| -12- |
T lymphocytes have long been known to play an important role in cancer suppression and modulation of tumor growth and numerous experimental studies have demonstrated the anti-cancer potential of GD-T lymphocytes. Indeed, GD-T cells can recognize a number of specific tumor-associated molecules including non-peptidic antigens (IPP’s – isopentenyl pyrophosphate) and immune surveillance stress signals (such as HSP60/70, MICA, MICB, and ULBP) present on the surface of transformed cells. The GD-T cell overexpresses IL-2 receptors and this cytokine is necessary to activate them (Kjeldsen-Kragh, 1993). On recognizing a tumor cell, GD-T cells exert their anti-cancer properties via release of both perforin and of granzyme, a serine protease which enters the target cell to trigger cell death (apoptosis). Our research efforts are focused entirely on targeting tumors in ways that may result in an improved therapeutic index and that have potential applications in solid tumors as well as hematological malignancies. In contrast to conventional AB CAR-T cells, our GD-T cell technology provides greater specificity in targeting tumors through recognition of IPP-expressing cells, whilst avoiding on-target, off-tumor effects on healthy tissue lacking in IPPs.
Liquid cancers
For cell therapies to be effective several parameters need to be addressed. These include (i) viability, (ii) homing to the tumor, (iii) persistence at the tumor, and (iv) target-specificity.
Use of unmodified GD-Ts to treat blood cancers addresses all the above factors. We believe that (i) we have demonstrated therapeutic cells remain viable when injected into the bloodstream of cancer patients; (ii) our research shows GD-Ts injected into the bloodstream remain in-situ; and (iii) they persist for up to 100 days after administration. Moreover, we believe we have demonstrated that certain late-stage blood cancer patients treated with multiple GD-T doses have shown significantly positive responses. These findings lead TCB to believe that all patients with similar blood cancers may respond to GD-T cell therapy in a positive manner.
Solid cancers
We believe that it may be necessary to use CAR-T technology (i) to maximize therapeutic cell homing into the solid tumor site, and (ii) to increase GD-T cell persistence by ‘tethering’ the cell to antigens present on the cancer cell surface.
In order to overcome toxicities seen with conventional CAR-T approaches, we believe that we have developed a ‘co-stimulatory’ GD-T CAR which will only attack and kill cancerous cells whilst leaving healthy cells unharmed. This is important as many of the current conventional CAR-T therapies cannot distinguish target antigens expressed on healthy cells from those on cancerous cells, which results in various pathologies, including cytokine release syndrome, that in some cases had led to patient death. Such targeting of health cells with conventional CAR-T makes their use in solid cancers difficult, as too much healthy tissue is likely to be destroyed as ‘collateral’ damage in the treatment process.
| -13- |
The diagram below illustrates how TCB’s approach works, using the innate receptors on the GD-T cell surface to act as a ‘safety switch’ – such receptors are generally not triggered by healthy cells, only by disease markers (IPP’s) on the surface of cancerous or virally infected cells.
| A | B |
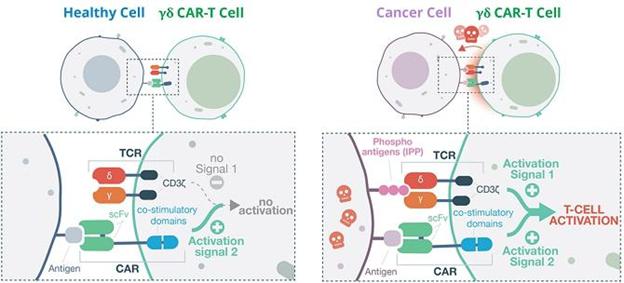 | |
Co-stimulatory CAR-T: A) No GD-T cell activation in healthy cell. B) GD-T activation and cell-killing in cancer cell.
Autologous cells are derived from ‘self’, using patients own cells to treat their specific disease
Allogeneic cells are derived from donor material, giving rise to cell banks able to treat numerous patients
Genetically-modified cells are typically engineered with a ‘chimeric’ receptor to target specific cancer antigens
Commercialization of conventional CAR-T cell therapy has taken decades of high-quality research in academia and industry, and it has provided transformational results for a number of patients with B cell malignancies. However, as noted, there are numerous barriers to widespread adoption, including:
| ● | Severe Toxicities. The significant risk of severe toxicities, especially cytokine release syndrome (CRS) and neurotoxicity occurring up to 3 weeks from treatment. These toxicities have resulted in the need for implementing specific clinical pathways to certify staff and facilities in the administration of the drugs and the management of the toxicities. | |
| ● | On-target, off tumor toxicities. Conventional CAR-T products have no mechanism for discriminating between diseased and healthy cells. Activation is governed solely by the expression of the target antigen, which can lead to toxicity when the target antigen is expressed on healthy cells. In marketed products targeting CD19 (present in the vast majority of B cells), this can be tolerated as B-cell aplasia, albeit with the need for regular long-term immunoglobulin replacement therapy. However, in experimental CAR-T products targeting other antigens this has been shown to cause serious side-effects, up to and including fatality. | |
| ● | Complex supply chains associated with autologous treatments. By definition, autologous treatments require the source cells to have been collected from the patient. It therefore requires a personalized supply chain with multiple touch points and the manufacturing process can only ever be performed on a single-patient batch size. This adds complexity to each treatment and has required the introduction of completely new processes and infrastructure in able to commercialize the products. | |
| ● | Inherent variability of the drug product. Each patient has a different cell population and so the starting material of each manufacturing batch is always variable, leading to variable final product. This can be minimized during pre-screening, which eliminates some patients from treatment, but there are still significant challenges in manufacturing to provide consistent batches of drug products and in understanding which variables are critical to product quality. |
| -14- |
| ● | High list price of the products. The need for personalized manufacturing, new supply chain processes and management of acute and chronic toxicities have all contributed to the high prices associated with the first CAR-T products reaching the market. In the USA, Kymriah® has a list price of $475,000 for pediatric ALL, and Yescarta® lists at $373,000 for DLBCL patients. The associated treatment costs and ongoing management can increase this price significantly. |
The combination of the co-stimulatory CAR, with GD-T cells, provides TCB with a proprietary platform which we believe addresses the problems with existing CAR-T products in the following ways:
| ● | Using the natural T cell signaling of the GD-T cell will, we believe, result in less risk of hyperactivation and tonic signaling with an overall reduction in the risk of CRS and less exhaustion of the cells. | |
| ● | The requirement on cell activation remains on the endogenous GD-T cell TCR signal, which detects stress signals associated with cancerous cells, so healthy cells are not targeted for destruction even if the target antigen is expressed and the CAR binds, thus off-tumor toxicity is avoided. | |
| ● | Manufacturing in batches of high dose numbers, without the complex patient collection of personalized supply chain steps, we believe will result in a dramatic reduction in cost of goods. This will be reflected in a list price which is in line with current biologicals. With the reduced likelihood of associated toxicities, the treatment and management costs should also be significantly lower, and the products can be made available to many more patients as a result. | |
| ● | The combination of a well-tolerated product and simplified supply chain (by virtue of our proprietary CryoTC freeze-thaw process), we believe, will make the therapy suitable for administration in local oncology centers without patients having to locate in centralized specialist centers of excellence, further reducing financial and logistic barriers to treatment. |
| ● | The tolerance of “off tumor” antigen binding without associated toxicity allows for a complete change in the current target identification paradigm. Instead of identifying targets that are exclusively expressed on tumor cells, we believe our co-stimulatory CAR-T approach confers an advantage to select targets that can be highly expressed on tumors and at low levels on healthy tissue. We select targets based on their relative therapeutic index increase in expression, their homogeneity in tumors and the antigen density. This allows us to target significantly more tumor associated antigens and to significantly expand the therapeutic index into higher doses or repeat administration. | |
| ● | GD-T cells have multiple roles in humans, possessing both innate and adaptive functions. One role is a sentinel surveillance cell, and they are biologically primed to travel through tissue searching for sites of cellular stress. This ability to penetrate tissue makes them advantageous agents for treating solid tumors. We can add additional function to the GD-T cells by using one or more co-stimulatory CAR-T constructs to add targeting to appropriate antigen(s) and to provide armor or strategies to overcome environmental and immune suppression in the tumor microenvironment. Therefore, we believe that the platform offers a promising approach to target the full spectrum of cancer diseases. |
Viral infections
GD-Ts are natural killers of virally infected cells, as well as cancerous cells. We believe that our unmodified GD-T therapy offers substantial potential as a first line of attack against future viral pandemics. During the COVID-19 pandemic, we took the opportunity to develop a trial protocol to treat patients with COVID-19, which was approved by the MHRA. We are currently not progressing this trial because of the absence of available patients given the progression of the disease; however we would consider conducting a phase 1b/2a trial if more severe/pathogenic variants emerge and we believe that there is considerable opportunity to deploy our GD-T therapy in the treatment of viral infections, including rapid response treatment of future epidemics and pandemics and selected acute viral infections. Whilst our current focus is to prioritize cancer treatment we will seek opportunities to develop viral treatments either on our own or in partnership in future Numerous peer-reviewed publications have demonstrated that GD-T cells innate killers of cells which have become virally infected. Using Epstein-Barr virus infected cells as an exemplar, TCB has conducted pre-clinical studies to demonstrate that our GMP-compliant manufacturing process results in GD-T with potent anti-viral cytotoxicity
| -15- |
Autologous versus allogeneic
Commercially available cell therapies typically are either autologous or allogeneic. Autologous products are taken from one donor (the patient) and used to treat that same donor (self-to-self), whilst allogeneic products are usually taken from a single donor (not a patient) and used as the starting material to treat a large number of different individuals (patients). GD-T lymphocytes are known to exert their biological effect in a non-MHC restricted manner. This means the potential for graft-versus-host mediated rejection is significantly reduced if allogeneic (non-self) cells are used as a treatment compared with many other immune cell therapies. As many patients with late-stage cancer or severe viral infections are also immunosuppressed, potential for host-mediated rejection of allogeneic cells is also reduced. When compared with autologous variants, commercial benefits of allogenic treatment include the following:
| ● | significant reduction in cost of goods; | |
| ● | product can be campaign manufactured and stockpiled frozen; | |
| ● | increased capacity to treat more patients; | |
| ● | logistics of shipping product are simplified; | |
| ● | higher doses of (reproducible) product are possible; and | |
| ● | product is immediately available for acute disorders |
Our strategy for developing an allogeneic solution for CAR-T is to select a pathway which will allow us to bring our products to patients as quickly as possible. These concepts build upon decades of previous development in allogeneic cell therapies and have clear understanding of development requirements in terms of manufacturing, clinical and regulatory execution.
Although manufacture of allogeneic cell therapies allows product to be “pharmaceuticalized” by virtue of campaign manufacture and storage, the approach is however not without technical and logistic challenges. To manufacture allogeneic banks, donor cells need to be screened for numerous adventitious agents, including for example, HIV, hepatitis, CMV and syphilis. Additional tumorgenicity testing is required, and assays conducted to ensure the cell bank is free from karyotypic aberrations. In order to overcome any potential for rejection, TCB has developed allogeneic GD-T cell banks that are unlikely to elicit a graft-versus-host (GvH) or host-versus-graft (HvG) immune response.
Donors are screened and selected based on clinically-relevant history and then based on the proliferative capacity and phenotypic character of their GD-Ts, based on a small volume blood draw and in-house assays. In this way, only good quality GD-T cells are selected for repeat apheresis and banking. The banks are HLA-typed and become the starting material for all of the allogeneic CAR-T products. These banks are cryopreserved in our facilities and can later be thawed, genetically engineered with the CAR, activated and expanded into final product, before being frozen again as multiple individual doses of drug product.
Generation of Gamma Delta T cells from IPSC cells
Identification of appropriate donors whilst possible is challenging as only a limited number of batches can be created from a single donation. GD-T cells can be routinely expanded from peripheral blood over 14 days. This provides a short window of opportunity for cell modification/engineering.
Induced pluripotent stem cells (iPSCs) have the potential to overcome these issues because they are capable of unlimited proliferation and multidirectional differentiation. In 2013, several research groups from Japan reported the successful reprogramming of αβT-cells, followed by re-differentiation back to αβT cells (Vizcardo et al., 2013; Nishimura et al., 2013; Themeli et al., 2013). While re-differentiated αβT cells-maintained antigen specificity, they were also characterized by higher proliferation ability than an original T-cell clone.
| -16- |
We hypothesized that GD-T derived iPSCs cells that carry the rearrangements at the TCRG and TCRD gene locus will be able to generate GD-T but not αβT cells. Furthermore, iPSC cells will provide a vast opportunity for the gene-editing without any time constraints of terminally differentiated cells.
Reprogramming GD-T cells has proven to be a challenge, as these cells are not tolerant of cell sorting. Therefore, GD-T cells can be reprogrammed in a bulk culture with the rest of peripheral blood cells or at the end of 14 days expansion, when the purity of GD-T is highest. After several unsuccessful reprogramming attempts, we have optimized the conditions favoring GD-T cells reprogramming. In the last round of reprogramming >50 clones were created. After extensive analysis of DNA rearrangements in δ- and γ-locus of 5 pre-selected clones, it was confirmed that they are derived from GD-T cells with different TCR sequences.
IPSC technology is an attractive approach for the limitless source of GD-T cells are successful progress in reprogramming has been demonstrated. Further work is now required for the establishment of a GMP compatible T-cell differentiation protocol. Generation of GDT cells from iPSC cells presents TCB with a vast opportunity for scaling without any time constraints of terminally differentiated cells.
Fresh versus frozen product
Commercial and clinical development of cellular therapy products will invariably require cryopreservation and frozen storage of cellular starting materials, intermediates and/or final product.
Optimizing cryopreservation is important to obtaining maximum yield and a consistent end-product. Suboptimal cryopreservation can lead not only to batch-to-batch variation, lowered cellular functionality and reduced cell yield, but also to the potential selection of subpopulations with genetic or epigenetic characteristics divergent from the original cell line.
Regulatory requirements also impact on cryopreservation, requiring a robust and reproducible approach to freezing, storage and thawing of the product. This requires attention to all aspects of the application of low temperatures; from the choice of freezing container and cryoprotectant, the cooling rate employed and its mode of delivery, correct handling of the frozen material during storage and transportation, to eventual thawing of the product by the end-user. Each of these elements influences all of the others to a greater or lesser extent and have been taken into consideration as TCB moves from fresh to cryopreserved cell-based product.
In a submission to UK regulators, we provided batch manufacture and supporting data, and TCB was granted approval to commence treatment of cancer patients using frozen allogeneic product. This represents a significant milestone for TCB, as we pioneer use of cryopreserved-donated cells to treat cancer. Obvious benefits include increased product reproducibility, ability to ship product globally on request and significant economy of scale (through batch manufacture and storage).
Clinical studies – unmodified GD-Ts in blood cancer
Management of acute myeloid leukemia (AML) is based on intensive chemotherapy and/or stem cell transplant, but these therapies lead to high relapse rates amongst treated patients. Particularly for the relapsed/refractory AML population or those who are not eligible for alloHSCT or intensive chemotherapy, the therapy options are limited, and patients are often placed in experimental protocol therapies or palliative care. As a result, there is a need for additional therapies, particularly for these cohorts.
GD-T cells have emerged as a promising therapy due to their ability to specifically target cancer cells. Nonclinical studies performed in AML cell lines suggest that GD-T cells specifically target AML tumor cells and lead to cell lysis in vitro (Kirk et al., 1993). Additionally, in xenotransplantation animal models, GD-T cells obtained from healthy volunteers specifically target AML cells and result in increased survival and diminished tumor burden in NOD mice (Gertner-Dardenne et al., 2012). Similarly, in vitro experiments conducted by TCB further support such findings whilst providing evidence that OmnImmune® (TCB002) specifically targets stress induced cells and effectively kills AML cells lines.
| -17- |
In the clinic, allogeneic treatment in AML patients in the phase 1b/2a trial OmnImmune® (TCB002) has shown our product is well-tolerated with some preliminary evidence of anticancer activity. Firstly, there were no signs of graft vs. host disease (GvHD) following therapy and secondly, CR (complete response) and MLFS (morphologic leukemia free state) were observed. Earlier results with autologous product demonstrated good tolerability. For the allogeneic product, OmnImmune® (TCB002), additional procedures were included to prevent GvHD (e.g. AB T cell depletion). Literature reports were also supportive of the use of OmnImmune® (TCB002) in cancer patients. The phase 1b/2a trial tested OmnImmune® (TCB002) in active relapsed or refractory AML who were not eligible for or did not consent to high dose salvage chemotherapy and/or allogeneic hematopoietic stem cell transplantation (alloHSCT). The trial was conducted to identify a tolerable dose and better understand the safety of this therapy in the chosen indication as well as generate preliminary information on potential clinical benefit. The primary, secondary and exploratory endpoints were as follows:
Primary endpoints:
| ● | Assessment of adverse events (Aes) graded by Common Terminology Criteria for Adverse Events (CTCAE) v5.0, vital signs and evaluation of laboratory parameters | |
| ● | Incidence of dose-limiting toxicities (DLTs) during the first 28 days after γδ T cell administration. | |
| ● | Establish Maximum Tolerated Dose (MTD) of OmnImmune® |
Secondary endpoints:
| ● | Complete Remission (CR) rate |
| ● | Overall survival (OS) | |
| ● | Quality of life determined by EORTC QLQ-C30 questionnaire |
Exploratory endpoints:
| ● | Changes in γδ T cell count and phenotype before and after OmnImmune® infusion |
No formal statistical analysis was planned. The incidence of DLTs were to be summarized descriptively by γδ T cells dose for evaluable patients. The recommended dose would be determined as the greatest with an incidence of DLTs no greater than 1/3. All other data including efficacy results were summarized descriptively by γδ T cells dose.
The trial enrolled 8 patients and healthy donors aged >18 years.
Clinical outcome
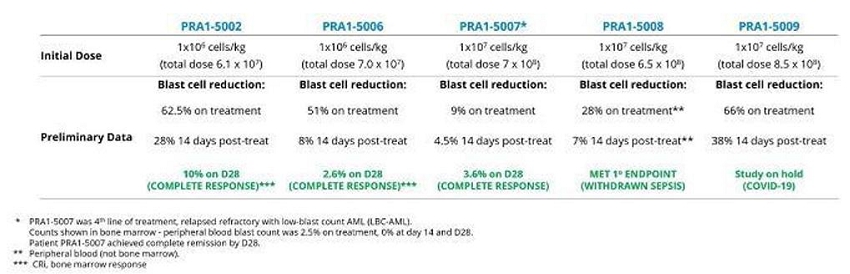
Seven patients were treated with OmnImmune® (TCB002). The eighth patient could not be dosed because the study was terminated as a result of the COVID-19 pandemic, which prevented the importation of investigational product from Scotland to the Czech Republic. No safety concerns were raised during Safety Review Committee (SRC) meetings. No treatment related Serious Adverse Reactions (SARs) were reported in any of the patients who were enrolled in the trial. No grade 3≥ OmnImmune® (TCB002) treatment related toxicities were noted in any of the treated patients. No dose-limiting toxicities were observed and no emergency safety measures have occurred for any subjects receiving OmnImmune® (TCB002). Two patients at 28 days post-treatment achieved a CR (one patient) or MLFS (one patient); another patient was classified as attaining stable disease with > 50% reduction in bone marrow blast count; one additional patient exhibited reduction in blast levels at 14 days; and one patient had disease progression (see table below). One patient (PRA1-5003) died 21 days after TCB002 due to bilateral pneumonia, determined unrelated to study medication. One patient (PRA1-5010) was withdrawn because of the COVD-19 pandemic before bone marrow aspiration on day 28 post-treatment. These preliminary indications of anticancer activity were not expected given the refractory profile of the enrolled patients.
| -18- |
The EORTC QLQ-C30 questionnaire resulted in scoring from six of the seven patients dosed with OmnImmune® (TCB002) for varying periods of time depending on their study duration. At 7 days post dosing, the average QoL score from six patients had decreased from 55.7 to 47.2 out of a possible maximum of 100. This negative impact on QoL reflects the well characterized side effects of preconditioning therapy with cyclophosphamide and fludarabine given between 6 and 2 days prior to OmnImmune® (TCB002) administration. The score remained lower in the four patients assessed at 28 days at a level of 50.0. In the two patients (one CR and one MLFS) who were assessed at the end of the study (week 24), both had recovered to an improved QoL score, each of 67.0.
FDA Orphan Drug Designation
About 60 million people living in the European Union (EU) and USA suffer from a rare disease. The European Medicines Agency (EMA) and FDA play a central role in facilitating the development and authorization of medicines for rare diseases, which are termed ‘orphan medicines’ in the medical world. Developing medicines intended for small numbers of patients has little commercial incentive under normal market conditions. Therefore, the EU and USA offer a range of incentives to encourage the development of designated orphan medicines.
The general therapeutic strategy for the treatment of AML has not changed substantially over the past 30 years. Excluding APL (which should be treated with trans-retinoic acid), AML management is based primarily on induction, incorporating an anthracycline and cytarabine, and consolidation therapy, and/or allogeneic Hematopoietic Stem Cell Transplantation (alloHSCT). Induction/consolidation therapy leads to high CRs rates in those who are eligible for treatment and present a favorable risk profile.
Several novel agents are in various stages of development for the treatment of AML. Novel approaches include antibody-based immunotherapy and adoptive cell therapy that aim to improve anti-leukemia T cell function, such as the therapies developed by TCB (OmnImmune®).
OmnImmune® (TCB002) was initially studied in patients with active relapsed or refractory AML who are not eligible or do not consent to high dose salvage chemotherapy and/or alloHSCT. In July 2019, OmnImmune® (TCB002) was granted ‘orphan medicine’ status from the FDA for Acute Myeloid Leukemia (AML). TCB intends to conduct a further clinical phase 2/3 study (OmnImmune® (TCB008-001)) in 2021/2 aimed at treating earlier stage AML patients.
| -19- |

Summary of TCB’s phase 1b/2a clinical trial in patients with fourth-line-of-treatment acute myeloid leukemia. Subsequent to the completion of this study TCB commenced phase 2b into 3 (pivotal) patient treatment during H1, 2022.
Pipeline and plan
Our future pipeline is focused on treating liquid cancers with our unmodified GD-T therapies and the treatment of solid cancers with next-generation allogeneic GD-T CAR-T therapies.
Our unmodified cell therapy, used in the treatment of Acute Myeloid Leukemia, is supplied under the name OmnImmune.
OmnImmune® is an allogeneic unmodified GD-T (GD-T2) cell product. Donor-derived GD-T cells for proliferative capacity, were activated and expanded in our manufacturing facility before being infused into the patient as part of our OmnImmune® (TCB002) phase 1 trial. This trial was completed in H1 2020 at the Institute of Hematology and Blood Transfusion in Prague, Czech Republic. Having generated meaningful clinical data showing our product is well-tolerated in late-stage AML patients with no remaining treatment options, TCB commenced a phase 2b-into pivotal (phase 3) clinical studies (with OmnImmune®) during 2022 in AML patients who have failed to respond adequately to induction therapy. The aim is to provide a form of salvage therapy which will either stabilize the patient, thereby preventing disease progression, or delay the requirement for human stem cell transplant. Our initial trial centers are in the UK. Working on the premise that other blood cancers should respond to GD-Ts in a similar manner to AML, TCB plans to conduct clinical studies for OmnImmune® in other hematological malignancies in future. The initial phase 1b/2a trials were undertaken using fresh cell-based product under the program number TCB002. For ease of reference, when discussing that specific trial, we refer the program as OmnImmune® (TCB002). The subsequent planned phase 2b-into pivotal (phase 3) clinical studies uses a frozen cell-based product under the program number TCB008-001. When discussing that specific trial, we refer the program as OmnImmune®.
We plan to develop a range of allogeneic co-stimulatory GD-T CAR pre-clinical drug candidates which will target antigens expressed on a number of solid tumor types.
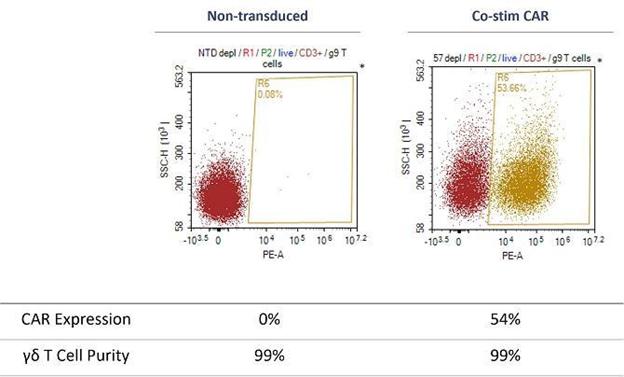
TCB has generated in-vitro preclinical data as part of our CAR-T program which demonstrated that GD-Ts are very high purity and can be CAR-transduced with high efficiency (see diagram below). Gamma delta cell purity and transduction efficiency have been measured using flow cytometry. CAR positive cells were measured by a detection reagent labelled with the fluorophore Phycoerythrin (PE). Flow cytometry analysis used the parameters of side scatter height (SSC-H) and PE area (PE-A) to define the cell populations. This is demonstrated in the figure below comparing non-transduced (NTD) and transduction with a co-stimulatory CAR construct (co-stim CAR).
| -20- |
We have also demonstrated that following transduction with different CAR constructs, GD-T’s can be effectively and reproducibly expanded in-vitro whilst exhibiting increased cytotoxicity in a zoledronate-dependent manner (see diagrams below – zoledronate-dependency reflects TCB’s proprietary process for commercial expansion of GD-T’s). The CAR constructs contained different endodomains including DNAX-activating protein 10 (DAP-10) and the high affinity IgE receptor (FcR) with no endodomain (no-endo) and non-transduced (NTD) as controls. These data outline the key preclinical parameters investigated in advance of progressing our CAR-T products into clinical trials. TCB has engaged with UK regulators to discuss the design of GD-T CAR phase1b/2a clinical studies (specifically relating to patient dosing and quality systems).
| -21- |
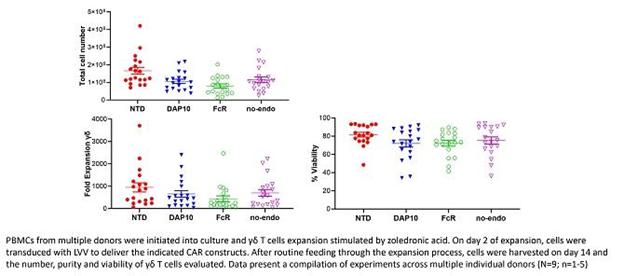
Peripheral blood mononuclear cells (PBMCs) were initiated into culture and GD-T cells expansion stimulated by zoledronic acid. On day 2 of expansion, cells were transduced with lentiviral vectors (LVV) to deliver the indicated CAR constructs. After routine feeding through the expansion process, cells were harvested on day 14 and the total cell number, fold expansion and viability of GD-T cells evaluated. Data present a compilation of experiments across multiple individual donors (N=9; n=1-5).
Corporate Information
Our principal executive offices are located in Scotland, United Kingdom, with a mailing address of Maxim 1, 2 Parklands Way, Holytown, Motherwell, ML1 4WR, United Kingdom and our telephone number at that location is +44 (0) 141 433 7557. Our website address is https://www.tcbiopharm.com. The information contained on, or that can be accessed through, our website is not part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
Implications of Being an “Emerging Growth Company”
We are an “emerging growth company,” as defined in Section 2(a) of the Securities Act of 1933, as amended, or the Securities Act. As such, we are eligible to, and intend to, take advantage of certain exemptions from various reporting requirements applicable to other public companies that are not “emerging growth companies” such as not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting of Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act. We could remain an “emerging growth company” for up to five years, or until the earliest of (a) the last day of the first fiscal year in which our annual gross revenue exceeds $1.235 billion, (b) the date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended, or the Exchange Act, which would occur if the market value of all our ordinary shares, including those represented by the ADSs, that are held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter, or (c) the date on which we have issued more than $1 billion in nonconvertible debt during the preceding three-year period.
Recent Developments
April 2024 LOI
On April 1, 2024, we entered into a non-binding letter of intent (the “Asset LOI”) with an unnamed cell therapy company. (the “Asset Seller”), regarding the potential acquisition (the “Proposed Asset Transaction”) by the Company of the following assets of Asset Seller: a Solid Tumor tool kit, a NK Cell Manufacturing tool kit, and two CAR-NK programs (the “Assets”). In exchange for the sale of the Assets to the Company, the Company will pay to the Asset Seller a combination of cash and equity at closing, as well as milestone payments based upon certain clinical achievements.
| -22- |
The Asset LOI only represents a mutual indication of interest regarding the Proposed Asset Transaction and the terms of the Proposed Asset Transaction are subject to a number of contingencies, including the completion of customary due diligence and the negotiation and execution of definitive agreements. Upon execution of the definitive agreements, the completion of the transaction will be subject to, among other matters, satisfaction of the conditions negotiated therein, the Company having secured adequate financing, and receipt of all third party (including governmental) approvals, licenses, consents, and clearances, as and when applicable. There can be no assurance that the Proposed Asset Transaction will be completed on the terms contemplated in the Asset LOI or otherwise. In particular, the timing of closing of any such transaction and the aggregate consideration that we may pay may materially differ from that currently contemplated by the Asset LOI.
May 2024 LOI
On May 1, 2024, we entered into a non-binding letter of intent (the “LOI”) with a private company (the “Seller”), regarding a potential business combination (the “Proposed Transaction”) whereby the Company or a subsidiary of the Company would acquire the Seller. In connection with the Proposed Transaction, the Company will pay to the Seller a cash purchase price equal to $20 million less any amounts payable on any Seller indebtedness and issue American Depository Shares (the “ADSs”) representing a number of the Company’s ordinary shares (the “Shares”) where the issue price of such Shares is equal to the average price paid in a fundraising from new and existing shareholders in the Company raising in excess of US$50 million (the “Issue Price”), such that the total value attributable to the Shares at closing is equal to US$20 million. In addition, the Seller will be entitled to certain payments upon satisfaction of various development milestones.
The LOI only represents a mutual indication of interest regarding the Proposed Transaction and the terms of the Proposed Transaction are subject to a number of contingencies, including the completion of customary due diligence and the negotiation and execution of definitive agreements. Upon execution of the definitive agreements, the completion of the transaction will be subject to, among other matters, satisfaction of the conditions negotiated therein, the Company having secured adequate financing, and receipt of all third party (including governmental) approvals, licenses, consents, and clearances, as and when applicable. There can be no assurance that the Proposed Transaction will be completed on the terms contemplated in the LOI or otherwise. In particular, the timing of closing of any such transaction and the aggregate consideration that we may pay may materially differ from that currently contemplated by the LOI.
May 2024 Warrant Inducement
On May 6, 2024, the Company, entered into a letter agreement (the “Inducement Letter”) with certain holders (the “Holders”) of existing Series E warrants (the “Existing Warrants”) to purchase ordinary shares represented by american depositary shares (the “ADSs”) of the Company. The Existing Warrants were issued on December 21, 2023 and have an exercise price of £1.5814 per ADS. Each ADS represents twenty (20) ordinary shares of the Company.
Pursuant to the Inducement Letter, the Holders agreed to exercise for cash their Existing Warrants to purchase an aggregate of 1,750,000 ADSs of the Company for cash and the payment of £0.099625 (US$0.125) per new warrant in consideration for the Company’s agreement to issue new Series F warrants to purchase ordinary shares represented by ADSs (the “New Warrants”), as described below, to purchase up to 70,000,000 of the Company’s ordinary shares represented by 3,500,000 ADSs (the “New Warrant ADSs”). The Company expects to receive aggregate gross proceeds of approximately £3.1 million from the exercise of the Existing Warrants by the Holders, prior to deducting placement agent fees and estimated offering expenses.
| -23- |
The Company engaged H.C. Wainwright & Co., LLC (the “Placement Agent”) to act as its exclusive placement agent in connection with the transactions summarized above and has agreed to pay the Placement Agent a cash fee equal to 7.5% of the gross proceeds received from the Holders’ exercise of their Existing Warrants and a management fee of 1% of the gross proceeds received from the Holders’ exercise of their Existing Warrants. The Company has also agreed to reimburse the Placement Agent for its expenses in connection with the exercise of the Existing Warrants and the issuance of the New Warrants, up to $50,000 for fees and expenses of legal counsel and other out-of-pocket expenses, and agreed to pay the Placement Agent for non-accountable expenses in the amount of $35,000 and a clearing fee of $15,950. Upon any exercise for cash of any New Warrants, the Company has agreed to pay the Placement Agent a cash fee of 7.5% of the aggregate gross exercise price paid in cash with respect the exercise of the New Warrants. In addition, the Company granted warrants (“Placement Agent Warrants”) to the Placement Agent, or its designees, to purchase up to an aggregate of 2,625,020 ordinary shares represented by 131,251 ADSs, which Placement Agent Warrants shall be substantially in the same form as the New Warrants except that the Placement Agent Warrants will have an exercise price of £2.2313.
The closing of the transactions contemplated pursuant to the Inducement Letter occurred on May 8, 2024. The Company intends to use the net proceeds from this offering to support its upcoming clinical trial focusing on relapse/refractory Acute Myeloid Leukemia, and for continuing operating expenses and working capital.
The Company also agreed to file a registration statement on Form S-3 (or other appropriate form if the Company is not then Form S-3 eligible) covering the resale of the New Warrant ADSs issued or issuable upon the exercise of the New Warrants (the “Resale Registration Statement”), within 30 days of the Closing Date, and to have such Resale Registration Statement declared effective by the SEC within 90 days following the Closing Date. The registration statement of which this prospectus is a part is being filed to fulfill our obligations under the Letter Agreement.
In the Inducement Letter, the Company agreed not to issue any ADSs, ordinary shares or ordinary share equivalents or to file any other registration statement with the SEC (in each case, subject to certain exceptions) until 30 days after the Closing Date. The Company also agreed not to effect or agree to effect any variable rate transaction (as defined in the Inducement Letter) until one (1) year after the Closing Date (subject to an exception).
Nasdaq Compliance
As previously reported in a Current Report on Form 8-K filed with the Securities and Exchange Commission (the “SEC”) on May 20, 2024 (the “May 20 8-K’), on May 15, 2024, the Company filed its Form 10-Q for the quarter ended March 31, 2024 (the “Form 10-Q”). As noted in the Form 10-Q, the Company was not in compliance with the minimum stockholders’ equity requirement under Nasdaq Listing Rule 5550(b)(1) for continued listing on The Nasdaq Capital Market because its stockholders’ equity was below the required minimum of $2.5 million (the “Minimum Stockholders’ Equity Requirement”) at March 31, 2024. As previously reported in a Current Report on Form 8-K filed with the SEC on May 8, 2024, on May 6, 2024, the Company entered into a letter agreement (the “Inducement Letter”) with certain holders (the “Holders”) of existing Series E warrants (the “Existing Warrants”) to purchase ordinary shares represented by american depositary shares (the “ADSs”) of the Company. Pursuant to the Inducement Letter, the Holders agreed to exercise for cash their Existing Warrants to purchase an aggregate of 1,750,000 ADSs of the Company for cash and the payment of £0.099625 (US$0.125) per new warrant in consideration for the Company’s agreement to issue new Series F warrants to purchase ordinary shares represented by ADSs (the “New Warrants”) to purchase up to 70,000,000 of the Company’s ordinary shares represented by 3,500,000 ADSs (the “New Warrant ADSs”). On May 8, 2024, the Company received aggregate gross proceeds of approximately £3.1 million (circa $3.9 million) from the exercise of the Existing Warrants by the Holders, prior to deducting placement agent fees and estimated offering expenses. As a result, the Company believes that due to the exercise of the Existing Warrants it is now in compliance with the Minimum Stockholders’ Equity Requirement.
On May 24, 2024, the Company received written notification from the listing qualifications staff of the Nasdaq Stock Market, LLC (“Nasdaq”) indicating that the Company was not in compliance with the Minimum Stockholders’ Equity Requirement, as of March 31, 2024. This letter indicated that while Nasdaq estimates the Company is currently in compliance with the Minimum Stockholders’ Equity Requirement it notes that based on the historical burn rate, without a significant transaction, the Company will not be in compliance as of the next period ending June 30, 2024.
Since the Company was previously granted an exception to the Minimum Stockholders Equity Requirement by a Nasdaq Hearings Panel and subsequently regained compliance, it is subject to a Mandatory Panel Monitor in accordance with Nasdaq Listing Rule 5815(d)(4)(A).
The Company has requested a hearing before a hearing panel at which it will request continued listing on The Nasdaq Capital Market since it has returned to compliance and expects to continue to do so. The Company’s hearing request will stay the suspension of trading and delisting of the Company’s ADSs and Warrants pending the conclusion of the hearing process. Consequently, the Company’s ADSs and Warrants will remain listed on The Nasdaq Capital Market at least until the hearing panel renders a decision following the hearing. There can be no assurance that the hearing panel will determine to continue the Company’s listing on The Nasdaq Capital Market or that the Company will timely evidence compliance with the terms of any extension that may be granted by the Nasdaq following the hearing.
| -24- |
The Offering
This prospectus relates to the resale by the selling shareholder identified in this prospectus of up to an aggregate of 3,500,000 ADSs (representing 70,000,000 ordinary shares) deliverable upon exercise of the Warrants. The selling shareholder may sell its ADSs from time to time at prevailing market prices. We will not receive any proceeds from the sale of the ADSs by the selling shareholder. However, we will receive cash proceeds equal to the total exercise price of any Warrants that are exercised for cash.
| ADSs, offered by Selling Shareholders | warrants to purchase up to 3,500,000 ADSs. See “Description of Securities.” | |
| ADSs | Each ADS represents twenty (20) ordinary shares. As a holder of ADSs, we will not treat you as one of our shareholders. The depositary, through its custodian, will be the holder of the ordinary shares underlying the ADSs, and you will have the rights of a holder of ADSs or beneficial owner (as applicable) as provided in the deposit agreement among us, the depositary and owners and holders of ADSs from time to time. To better understand the terms of the ADSs we encourage you to read the deposit agreement, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part. | |
| Warrants | Each Warrant will be immediately exercisable, will expire three and one-half (3.5) years from the date of issuance, or November 8, 2027 and have an exercise price of £1.175 per ADS ($1.469 per ADS translated for illustration to U.S. dollars at the rate of £1.00 to $1.2503), subject to adjustment as set forth therein. | |
| Ordinary shares outstanding before this offering | 98,902,641 ordinary shares | |
| Warrants outstanding before this offering | Warrants to purchase 338,169 ADSs
| |
| Ordinary shares to be outstanding after this offering, including ordinary shares represented by ADSs | 168,902,641 ordinary shares | |
| Use of proceeds | We will not receive any of the proceeds from the sale of ADSs by the Selling Shareholder pursuant to this prospectus. However, we will receive the proceeds of any cash exercise of the Warrants. The Selling Shareholder will pay any agent’s commissions and expenses they incur for brokerage, accounting, tax or legal services or any other expenses that they incur in disposing of the ADSs. We will bear all other costs, fees and expenses incurred in effecting the registration of the ADSs covered by this prospectus and any prospectus supplement. | |
| Risk factors | See “Risk Factors” beginning on page 28 of this prospectus, as well as other information included in this prospectus, for a discussion of factors you should read and consider carefully before investing in our securities. | |
| Nasdaq Capital market symbols | ADSs on the Nasdaq Capital Market under the symbol “TCBP.” |
| -25- |
The number of our ordinary shares (including shares represented by ADSs) to be outstanding after this offering is based on 168,902,641 ordinary shares outstanding as of June 19, 2024 and excludes:
| ● | 106,585 ordinary shares issuable upon the exercise of options outstanding under our 2014 Share Option Scheme as of March 31, 2024, with a weighted-average exercise price of £23.00 per share; | |
| ● | 20,202 ordinary shares issuable upon the exercise of options outstanding under our 2021 Share Option Scheme, as of March 31, 2024, with a weighted-average exercise price of $212.00 per share; | |
| ● | 17,575,360 ordinary shares issuable upon the exercise of options outstanding under our 2021 Share Option Scheme, as of March 31, 2024, with a weighted-average exercise price of $0.06 per share; | |
| ● | 702,500 ordinary shares issuable upon the exercise of options outstanding under our 2021 Share Option Scheme, as of March 31, 2024, with a weighted-average exercise price of $0.409 per share; | |
| ● | 15,891 ordinary shares issuable upon the exercise of options outstanding, at a future date based on the achievement of certain clinical and commercial milestones with an exercise price of £215.00 per share; and | |
| ● | 6,763,370 ordinary shares issuable upon the exercise of warrants outstanding, as of May 31, 2024, with a weighted-average exercise price of £1.476 per share. |
For the description of the 2014 Share Option Scheme and 2021 Share Option Scheme please refer to the 2023 Form 10-K, which is incorporated by reference herein.
Unless otherwise stated, all information in this prospectus assumes no exercise of the outstanding options described above into ordinary shares or ADSs, treats all restricted shares issued with outstanding restrictions to be vested as issued and outstanding shares, no exercise of the Placement Agent Warrants issued in this offering and no sale of pre-funded warrants in this offering.
Except as otherwise indicated all references to our articles of association in this prospectus refer to our articles of association, as amended as currently in force for TC BioPharm (Holdings) plc at the date of this prospectus.
Summary Consolidated Financial Data
The following table summarizes our consolidated financial data as at the dates and for the periods indicated. The consolidated financial statement data as at December 31, 2023 and 2022, and for the years ended December 31, 2023 and 2022 audited in accordance with the standards of the Public Company Accounting Oversight Board (United States) have been derived from our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”).
Our historical results are not necessarily indicative of the results that may be expected in the future.
This information should be read together with, and is qualified in its entirety by, our consolidated financial statements and the notes thereto. You should read the following summary consolidated financial and other data in conjunction with “Item 5. Operating and Financial Review and Prospects” and Item 8 (“Financial Information’), our consolidated financial statements and the notes thereto and the other financial information included in our 2023 Form 10-K annual report and incorporated by reference in this prospectus.
| -26- |
| Consolidated Statement of Operations: | For the Year Ended | For the Year Ended | ||||||
| December 31, 2023 | December 31, 2022 | |||||||
| Revenue | £ | - | £ | 3,844,532 | ||||
| Operating expenses: | ||||||||
| Research and development expenses | 7,771,391 | 7,592,470 | ||||||
| Administrative expenses | 6,467,932 | 7,030,972 | ||||||
| Administrative expenses - costs related to preparing for listing | - | 1,305,087 | ||||||
| Total operating expenses | 14,239,323 | 15,928,529 | ||||||
| Loss from operations | (14,239,323 | ) | (12,083,997 | ) | ||||
| Other income (expense): | ||||||||
| Loss on modification of convertible loan | (645,845 | ) | (140,344 | ) | ||||
| Change in fair value of derivative liability | 8,052,581 | 16,064,945 | ||||||
| Foreign currency losses | (80,070 | ) | (120,974 | ) | ||||
| Interest expense | (83,025 | ) | (6,753,231 | ) | ||||
| Total other income (expense), net | 7,243,641 | 9,050,396 | ||||||
| Net loss before income taxes | (6,995,682 | ) | (3,033,601 | ) | ||||
| Income tax credit | 1,088,729 | 1,720,000 | ||||||
| Net loss | £ | (5,906,953 | ) | £ | (1,313,601 | ) | ||
| Weighted-average ordinary shares outstanding, basic and diluted (1) | 6,178,423 | 687,199 | ||||||
| Basic and diluted net loss per share (1) | £ | (0.96 | ) | £ | (1.91 | ) | ||
| Consolidated Statement of Financial Position items: | December 31, 2023 | December 31, 2022 | ||||||
| Cash and cash equivalents | £ | 2,462,609 | £ | 4,808,060 | ||||
| Working capital (2) | 950,326 | (1,716,361 | ) | |||||
| Total assets | 8,931,664 | 11,291,977 | ||||||
| Total liabilities | 6,246,434 | 10,960,712 | ||||||
| Share capital | 399,455 | 397,493 | ||||||
| Additional paid-in capital | 41,123,065 | 33,308,568 | ||||||
| Accumulated deficit | (38,837,290 | ) | (33,374,796 | ) | ||||
| Total shareholders’ equity | 2,685,230 | 331,265 | ||||||
| (1) | On November 18, 2022, the Company completed a reverse stock split of one (1) new share for every fifty (50) existing shares effective November 21, 2022. As a result of the share split, all references in these financial statements and accompanying notes to units of ordinary shares or per share amounts are reflective of the reverse share split for all periods presented. In addition, the exercise prices and the numbers of ordinary shares issuable upon the exercise of any outstanding options to purchase ordinary shares were proportionally adjusted pursuant to the respective anti-dilution terms of the share-based payment plans. |
| (2) | Working capital is defined as current assets less current liabilities. |
| -27- |
Our business is subject to a number of risks and uncertainties, including those risks discussed at length in Item 3D (“Risk Factors”) in our 2023 Form 10-K incorporated into this prospectus by reference. These risks include among others those summarized below. Investing in our company and its securities involves a high degree of risk. You should carefully consider the risks and uncertainties described below, together with all of the other information in this prospectus, including the information incorporated by reference to our 2023 Form 10-K, before investing in our company and our securities. If any of these risks materialize, our business, financial condition, operating results and prospects could be materially and adversely affected. In that event, the price or value of our ADSs in the public market could decline, and you could lose part or all of your investment.
The following is a summary of some of the principal risks we face. The list below is not exhaustive, and investors should read the risks described under the heading “Risk Factors” in our 2023 Form 10-K incorporated by reference herein, as well as the additional risks set forth in this section, in full.
| ● | We have generated operating losses since inception and expect to continue to generate losses. We may never achieve or maintain profitability. We will continue to require financing to continue to implement our business plan and sustain operations. | |
| ● | We, as well as our independent registered public accounting firm, in relation to our financial position, have expressed substantial doubt about our ability to continue as a going concern. | |
| ● | Our lack of any approved products and our limited operating history may make it difficult for an investor to evaluate the success of our business to date and to assess our future viability. | |
| ● | GD-T cell therapies are a novel approach to treating cancers and infectious diseases, which have development risks and will require us to obtain regulatory approvals for development, testing, commercialization, manufacturing and distribution. We may not achieve all the required regulatory approvals or approvals may not be obtained as timely as needed. |
| ● | Because GD-T cell therapies are a novel approach, potential side effects, and long-term efficacy, regulatory approval will require considerable time for trials, data collection, regulatory submissions and funding for the process. | |
| ● | Enrolling patients in clinical trials may be difficult for many reasons, including high screen failure, GD-T cell proliferation capacity, timing, proximity and availability of clinical sites, perceived risks, and publicity about the success or lack of success in the methods of treatment. | |
| ● | Because GD-T cell therapies are novel, our research and development and clinical trial results may not support our products intended purposes and regulatory approval. We are heavily dependent on the success of our lead product candidate (OmnImmune®), and intend to seek breakthrough therapy designation for some or all of our other therapeutic candidates in due course. | |
| ● | Market opportunities for certain of our product candidates may be limited to those patients who are ineligible for or have failed prior treatments. This class of patient may be limited in number, difficult to locate and service, require special governmental approval, and unable to pay or obtain reimbursement. | |
| ● | We rely on many third parties for aspects of our product development and commercialization, such as raw material supply, clinical trials, obtaining approvals, aspects of manufacturing, development of additional product candidates and distribution. We may not be able to control these parties and their business practices, such as compliance with good manufacturing requirements or their ability to supply or service us timely, which will likely disrupt our business. | |
| ● | We face substantial competition: others may discover, develop and/or commercialize competing products before or more successfully than TCB. |
| ● | Even if we are able to commercialize any product candidates, such drugs may become subject to unfavorable pricing regulations or third-party coverage and reimbursement policies. Commercialized products may not be adopted by the medical profession. | |
| ● | Because we operate internationally, we are subject to a wide array of regulation of the United Kingdom, European Union and United States. In addition to regulation surrounding new drug development and their manufacture, distribution and use, we will be subject, for example to data protection rules relating to medical records, medical and general privacy laws, environmental laws regarding medical waste, and bribery and corrupt practices law, in addition to all the drug related approval, manufacturing and distribution rules. |
| -28- |
| ● | Product liability claims are frequent in drug development of novel therapies and insurance is mandatory and expensive. The inability to obtain insurance may prevent product development and claims may surpass our ability to pay and call into question the efficacy of a product with resulting reputational damage. | |
| ● | Protecting our intellectual property is paramount in our ability to be able to commercialize our products and generate revenues and investment return for our stockholders. We may not be able to obtain the intellectual property protection we seek due to its cost, requirement to pursue it in many jurisdictions, challenges by others and patent office rejection. | |
| ● | Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies acting in multiple jurisdictions, and our patent protection could be reduced or eliminated for non-compliance with these requirements. | |
| ● | As part of product development, we may need to license aspects of our research and products from third parties or if our IP is challenged, we may have to seek license accommodation, any of which may be expensive, limited in scope, or unavailable. | |
| ● | We currently have a limited number of employees, and our future success depends on our ability to retain key executives and to attract, retain and motivate qualified personnel at all levels. |
| ● | We will need to grow the size and capabilities of our organization, and we may experience difficulties in managing this growth including, but not limited to, operating as a public company and taking a therapeutic through to market approval and acceptance. | |
| ● | We expect to expand our development and regulatory capabilities and potentially implement sales, marketing and distribution capabilities, and as a result, we may encounter difficulties in achieving and managing our growth, which could disrupt our operations. We expect to require further funding for these expansions of activity. | |
| ● | We incur substantial costs as a result of operating as a public company in the United States, and our management is required to devote substantial time to required SEC compliance and corporate governance practices. | |
| ● | If we fail to maintain proper and effective internal controls, our ability to produce accurate financial statements on a timely basis could be impaired, which would adversely affect our business and our stock price. | |
| ● | Certain of our existing shareholders, members of our board of directors and senior management maintain the ability to exercise significant control over us. The interests of investors may conflict with the interests of these other stockholders. | |
| ● | Our ADSs provide rights that are different from directly holding our ordinary shares. The outstanding warrants do not have the rights of shareholders until exercised. Our warrants form a substantial part of our capitalization, and they have substantial protective provisions, which may limit our ability to raise capital. | |
| ● | Future sales, or the possibility of future sales, of a substantial number of our ordinary shares, through the additional deposit of ordinary shares for ADSs, issuances and/or exercises of our warrants, could adversely affect the price of our ADSs or warrants in the market. After any lock up period, a substantial number of our issued and outstanding ordinary shares will be eligible for trading on the public securities market by their being deposited with the depositary for ADSs. | |
| ● | Shareholder rights and recourse will be governed by and ultimately determined by Scottish and United Kingdom law and judicial process, which in many ways are more limited than United States law and practice. Most of our assets are located in the United Kingdom. | |
| ● | If we fail to meet the requirements for continued listing on Nasdaq, our ADSs could be delisted from trading, which would decrease the liquidity of our ADSs and our ability to raise additional capital. |
| -29- |
Risks Related to this Offering and Ownership of ADSs
The price of the ADSs has been, and is likely to continue to be, highly volatile, which could result in substantial losses for purchases of ADSs in this offering.
The price of the ADSs has been, and is likely to continue to be, highly volatile. The stock market in general and the market for smaller pharmaceutical and biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, purchasers of securities sold pursuant to this registration statement may not be able to sell their ADSs at or above the price paid by such purchasers and, as such, they may lose some or all of their investment. Additionally, in the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us in light of the significant stock price volatility we and other pharmaceutical companies have experienced in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
We have broad discretion in the use of the net proceeds from this offering and any exercise of the Warrants and consequently may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from this offering and any exercise of any Warrant and could spend any such proceeds in ways that do not improve our results of operations or enhance the value of our ADSs. The failure by our management to apply these funds effectively could result in financial losses that could cause the price of our ADSs to decline and delay the development of our product candidates.
If we fail to meet the requirements for continued listing on the Nasdaq Global Market or Nasdaq, our ADSs could be delisted from trading, which would decrease the liquidity of our ADSs and our ability to raise additional capital.
Our ADSs are currently listed for quotation on The Nasdaq Capital Market. We are required to meet specified financial requirements in order to maintain our listing on the Nasdaq Capital Market. These requirements include maintaining a minimum bid price of at least $1.00 per share for our ADSs, which is referred to as the Bid Price Rule, and maintaining a minimum market value of listed securities, or the MVLS, of $35,000,000. On July 12 and 15, 2022, we received deficiency letters from the Listings Qualifications Department of the Nasdaq Stock Market notifying that we were not in compliance with the Bid Price Rule and the MVLS, respectively.
On December 6, 2022, we received written notification from the listing qualifications staff of the Nasdaq Stock Market, LLC (“Nasdaq”) indicating that the Company regained compliance with the Bid Price Rule. On January 12, 2023, we received written notification from the listing qualifications staff of the Nasdaq indicating that we have not regained compliance with the MVLS and that our securities would be subject to delisting unless we timely request a hearing before a Nasdaq Hearings Panel (the “Panel”). On March 9, 2023 the Company presented a formal plan to regain compliance to the Panel. On March 17, 2023, the Company announced that the TC BioPharm (Holdings) plc has been granted a formal extension until June 30, 2023, to regain compliance under Nasdaq Listing Rule 5550(b)(2) or its alternative criteria. The Company informed the Panel of its intention to regain compliance with Nasdaq’s continued listing requirements by demonstrating compliance with the $2.5m minimum stockholders’ equity requirement in Listing Rule 5550(b)(1) as an alternative to demonstrating compliance with the MVLS Requirement, the Panel granted the Company an exception until June 30, 2023. On July 27, 2023, the Company received a letter, dated July 26, 2023 (the “Letter”) from Nasdaq notifying the Company that the Panel has concluded that the Company has regained compliance with Nasdaq’s continued listing requirements. The Letter stated that, pursuant to Listing Rule 5815(d)(4)(A), the Company will be subject to a Panel Monitor for a period of one year from the date of the Letter. If, within that one-year monitoring period, the Listing Qualifications staff (the “Staff”) finds the Company again out of compliance with any continued listing requirement, notwithstanding Rule 5810(c)(2), the Company will not be permitted to provide the Staff with a plan of compliance with respect to any deficiency and the Staff will not be permitted to grant additional time for the Company to regain compliance with respect to any deficiency, nor will the Company be afforded an applicable cure or compliance period. Instead, the Staff will issue a Delist Determination Letter and the Company will have an opportunity to request a new hearing with the initial Panel or a newly convened Hearings Panel if the initial Panel is unavailable.
| -30- |
On June 22, 2023, we received a deficiency letter from the Staff notifying that we again were not in compliance with the Bid Price Rule. We have been provided an initial period of 180 calendar days, or until December 19, 2023, to regain compliance with the applicable listing requirement. On December 28, 2023, we received a letter from Nasdaq indicating that it has not regained compliance with the rule and we were not eligible for a second 180 day period. On January 2, 2024, we received written confirmation from Nasdaq that it has determined that for the last 10 consecutive business days, from December 15, 2023 to December 29, 2023, the closing bid price of the Company’s securities has been at $1.00 per share or greater. Accordingly, the Company has regained compliance with Listing Rule 5550(a)(2) and the matter is now closed.
As previously reported in a Current Report on Form 8-K filed with SEC on May 20, 2024 (the “May 20 8-K’), on May 15, 2024, the Company filed its Form 10-Q for the quarter ended March 31, 2024 (the “Form 10-Q”). As noted in the Form 10-Q, the Company was not in compliance with the minimum stockholders’ equity requirement under Nasdaq Listing Rule 5550(b)(1) for continued listing on The Nasdaq Capital Market because its stockholders’ equity was below the required minimum of $2.5 million (the “Minimum Stockholders’ Equity Requirement”) at March 31, 2024. As previously reported in a Current Report on Form 8-K filed with the SEC on May 8, 2024, on May 6, 2024, the Company entered into a letter agreement (the “Inducement Letter”) with certain holders (the “Holders”) of existing Series E warrants (the “Existing Warrants”) to purchase ordinary shares represented by american depositary shares (the “ADSs”) of the Company. Pursuant to the Inducement Letter, the Holders agreed to exercise for cash their Existing Warrants to purchase an aggregate of 1,750,000 ADSs of the Company for cash and the payment of £0.099625 (US$0.125) per new warrant in consideration for the Company’s agreement to issue new Series F warrants to purchase ordinary shares represented by ADSs (the “New Warrants”) to purchase up to 70,000,000 of the Company’s ordinary shares represented by 3,500,000 ADSs (the “New Warrant ADSs”). On May 8, 2024, the Company received aggregate gross proceeds of approximately £3.1 million (circa $3.9m) from the exercise of the Existing Warrants by the Holders, prior to deducting placement agent fees and estimated offering expenses. As a result, the Company believes that due to the exercise of the Existing Warrants it is now in compliance with the Minimum Stockholders’ Equity Requirement.
On May 24, 2024, the Company received written notification from the listing qualifications staff of the Nasdaq indicating that the Company was not in compliance with the Minimum Stockholders’ Equity Requirement, as of March 31, 2024. This letter indicated that while Nasdaq estimates the Company is currently in compliance with the Minimum Stockholders’ Equity Requirement it notes that based on the historical burn rate, without a significant transaction, the Company will not be in compliance as of the next period ending June 30, 2024.
Since the Company was previously granted an exception to the Minimum Stockholders Equity Requirement by a Nasdaq Hearings Panel and subsequently regained compliance, it is subject to a Mandatory Panel Monitor in accordance with Nasdaq Listing Rule 5815(d)(4)(A).
The Company has requested a hearing before a hearing panel at which it will request continued listing on The Nasdaq Capital Market since it has returned to compliance and expects to continue to do so. The Company’s hearing request will stay the suspension of trading and delisting of the Company’s ADSs and Warrants pending the conclusion of the hearing process. Consequently, the Company’s ADSs and Warrants will remain listed on The Nasdaq Capital Market at least until the hearing panel renders a decision following the hearing. There can be no assurance that the hearing panel will determine to continue the Company’s listing on The Nasdaq Capital Market or that the Company will timely evidence compliance with the terms of any extension that may be granted by the Nasdaq following the hearing.
The Company continues to execute its business plan and is looking into various options available to regain compliance with Nasdaq’s continued listing standards and maintain its continued listing on the Nasdaq Capital Market. However, there can be no assurance that the Company will be able to maintain compliance with the Nasdaq listing rules. In addition, there can be no assurance that the Panel will determine to continue the Company’s listing on The Nasdaq Capital Market following the hearing.
The exercise of outstanding ADS purchase warrants and share options will have a dilutive effect on the percentage ownership of our capital stock by existing stockholders.
As of June 19, 2024, we had outstanding warrants to acquire 338,169 ADSs, and share options to purchase 18,420,538 shares of our ordinary shares. A significant number of such warrants have exercise prices above our ADSs’ recent trading prices, but the holders have the right, in certain circumstances, to effect a cashless exercise of such warrants. If a significant number of such warrants and share options are exercised by the holders, the percentage of our ADSs owned by our existing ADS holders will be diluted.
We face risks and uncertainties related to litigation, regulatory actions and government investigations and inquiries.
We are subject to, and may become a party to, litigation, claims, suits, regulatory actions and government investigations and inquiries.
The outcome of any litigation, regardless of its merits, is inherently uncertain. Any claims and lawsuits, and the disposition of such claims and lawsuits, could be time-consuming and expensive to resolve, divert management attention and resources, and lead to attempts on the part of other parties to pursue similar claims. Negative perceptions of our business may result in additional regulation, enforcement actions by the government and increased litigation, or harm to our ability to attract or retain customers or strategic partners, any of which may affect our business. Any damage to our reputation, including from publicity from legal proceedings against us or companies that work within our industry, governmental proceedings, unfavorable media coverage or class action could adversely affect our business, financial condition and results of operations.
An unfavorable outcome or settlement or any other legal, administrative and regulatory proceeding may result in a material adverse impact on our business, results of operations, financial position and overall trends. In addition, regardless of the outcome, litigation can be costly, time-consuming, and disruptive to our operations. Any claims or litigation, even if fully indemnified or insured, could damage our reputation and make it more difficult to compete effectively or to obtain adequate insurance in the future.
The Company has received a lawsuit asserting, among other things, breach of contract under the terms of certain convertible promissory notes. The lawsuit is pending before the High Court in England, and the Company has retained English legal representatives to defend it. The company does not believe that the outcome of the claims is likely to be material to the balance sheet of TC BioPharm (Holdings) plc.
| -31- |
If we fail to maintain proper and effective internal controls, our ability to produce accurate financial statements on a timely basis could be impaired, which would adversely affect our business and our stock price.
Ensuring that we have adequate internal financial and accounting controls and procedures in place to produce accurate financial statements on a timely basis is a costly and time-consuming effort that needs to be re-evaluated frequently. We may discover material weaknesses in our internal financial and accounting controls and procedures that need improvement from time to time.
Management is responsible for establishing and maintaining adequate internal control over financial reporting to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes. Management does not expect that our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within our company will have been detected.
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, we are required to furnish a report by our senior management on our internal control over financial reporting, commencing with our second annual report. However, while we remain an EGC we are not required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To prepare for eventual compliance with Section 404, once we no longer qualify as an EGC, we are engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants, adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented, and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, within the prescribed timeframe or at all, that our internal control over financial reporting is effective as required by Section 404. If we identify one or more material weaknesses, it could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements. In addition, if we are unable to produce accurate financial statements on a timely basis, investors could lose confidence in the reliability of our financial statements, which could cause the market price of either of our ADSs or Warrants, or both, to decline and make it more difficult for us to finance our operations and growth.
The Company notes that the auditors identified that the Company experienced difficulty in the accounting for complex financial instruments and leases, and the Company lacked adequate internal control over the account and assessment of complex financial instruments following control deficiencies which they assessed to be a material weakness in the Company’s internal control over financial reporting as of December 31, 2023. The Company recognizes these errors as a material weakness and has established controls to support assessment and review of accounting for complex financial instruments and leases.
Since inception, we have not declared or paid any dividends on our ordinary shares. We do not have any current plans to pay any dividends on our ordinary shares, including those represented by ADSs, in the foreseeable future. We intend to retain all our available funds and any future earnings to operate and expand our business. Because we do not anticipate paying any cash dividends in the foreseeable future. Capital appreciation, if any, will be your sole source of gains, and you may never receive a return on your investment.
The determination to pay dividends, if any, will be made at the discretion of our board of directors and may be based on a number of factors, including our future operations and earnings, capital requirements and surplus, general financial condition, contractual and legal restrictions and other factors that the board of directors may deem relevant.
| -32- |
Under current Scottish law, among other things, a company’s accumulated realized profits must exceed its accumulated realized losses (on a non-consolidated basis) before dividends can be paid. Accordingly, we may only pay dividends if we have sufficient distributable reserves (on a non-consolidated basis), which are our accumulated realized profits that have not been previously distributed or capitalized less our accumulated realized losses, so far as such losses have not been previously written off in a reduction or reorganization of capital.
We will not receive any proceeds from the sale by the selling shareholder of the ADSs registered hereby or the shares underlying such ADSs. All net proceeds from the sale of the shares represented by ADSs will go to the selling shareholder.
We may receive proceeds from the exercise of the Warrants to the extent the warrants are exercised. We can make no assurances that any of the Warrants will be exercised, or if exercised, the quantity that will be exercised or the period in which such Warrants will be exercised.
We intend to use the net proceeds from any exercise of the Warrants for cash, together with our cash on hand, to advance our preclinical and clinical pipeline.
Our management will have broad discretion over the use of the net proceeds from any exercise of the Warrants for cash. The amounts and timing of our expenditures will depend upon numerous factors, including the results of our research and development efforts, the timing, cost and success of preclinical studies and ongoing clinical trials or clinical trials we may commence in the future, the timing of regulatory submissions, our ability to obtain additional financing, the amount of cash obtained through our existing collaborations and future collaborations, if any, and any unforeseen cash needs.
Pending any use described above, we may invest any proceeds from the exercise of any Warrants for cash in short- and intermediate-term interest-bearing obligations, investment-grade instruments, certificates of deposit or guaranteed government obligations.
MATERIAL INCOME TAX CONSIDERATIONS
The following summary contains a description of material U.K. and U.S. federal income tax consequences of the acquisition, ownership and disposition of our ordinary shares. This summary should not be considered a comprehensive description of all the tax considerations that may be relevant to the decision to acquire our ordinary shares.
U.S. Federal Income Taxes
The following is a summary of the material U.S. federal income tax consequences to U.S. Holders (as defined below) of purchasing, owing and disposing of the ordinary shares or ADSs. This discussion is included for general informational purposes only, does not purport to consider all aspects of U.S. federal income taxation that might be relevant to a U.S. Holder, and does not constitute, and is not, a tax opinion for or tax advice to any particular U.S. Holder of ordinary shares or the ADSs. The summary does not address any U.S. tax matters other than those specifically discussed. The summary is based on the provisions of the U.S. Internal Revenue Code of 1986, as amended (the “Code”), existing, temporary and proposed Treasury Regulations issued thereunder, judicial decisions and administrative rulings and pronouncements and other legal authorities, all as of the date hereof and all of which are subject to change, possibly with retroactive effect. Any such change could alter the tax consequences described herein.
The discussion below applies only to U.S Holders as capital assets within the meaning of Section 1221 of the Code (generally, property held for investment), and does not address the tax consequences that may be relevant to U.S. Holders who, in light of their particular circumstances, may be subject to special tax rules, including without limitation:
| ● | insurance companies, tax-exempt organizations, regulated investment companies, real estate investment trusts, brokers or dealers in securities or foreign currencies, banks and other financial institutions, mutual funds, retirement plans, traders in securities that elect to mark to market, certain former U.S. citizens or long-term residents; |
| -33- |
| ● | U.S. Holders that are classified for U.S. federal income tax purposes as partnerships and other pass-through entities and investors therein; |
| ● | U.S. Holders who hold ordinary shares or ADSs as part of a hedge, straddle, constructive sale, conversion, or other integrated or risk-reduction transaction, as “qualified small business stock,” within the meaning of Section 1202 of the Code or as Section 1244 stock for purposes of the Code; |
| ● | U.S. Holders who hold ordinary shares or ADSs through individual retirement or other tax-deferred accounts; |
| ● | U.S. Holders that have a functional currency other than the U.S. dollar; |
| ● | U.S. Holders who are subject to the alternative minimum tax provisions of the Code or the Medicare surtax of 3.8% on net investment income imposed by Section 1411 of the Code; |
| ● | U.S. Holders who acquire their ordinary shares or ADSs pursuant to any employee share option or otherwise as compensation; |
| ● | U.S. Holders required to accelerate the recognition of any item of gross income with respect to their ordinary shares or ADSs as a result of such income being recognized on an applicable financial statement; or |
| ● | U.S. Holders who hold or held, directly or indirectly, or are treated as holding or having held under applicable constructive attribution rules, 10% or more of the ordinary shares or ADSs of the company, measured by voting power or value. |
Any such U.S. Holders should consult their own tax advisors.
For purposes of this discussion, a “U.S. Holder” means a holder of our ordinary shares or ADSs that is or is treated as, for U.S. federal income tax purposes,
| (i) | an individual citizen or resident of the United States; |
| (ii) | a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any State thereof or the District of Columbia or any entity treated as such for U.S. federal income tax purposes; |
| (iii) | an estate the income of which is subject to U.S. federal income taxation regardless of its source, or |
| (iv) | a trust (A) the administration over which a U.S. court exercises primary supervision and all of the substantial decisions of which one or more U.S. persons have the authority to control, or (B) that has a valid election in effect under the applicable Treasury Regulations to be treated as a U.S. person under the Code. |
If a partnership or other pass-through entity (including any entity or arrangement treated as such for purposes of U.S. federal income tax law) holds our ordinary shares or ADSs, the tax treatment of a partner of such partnership or member of such entity will generally depend upon the status of the partner and the activities of the partnership. Partnerships and other pass-through entities holding our ordinary shares or ADSs, and any person who is a partner or member of such entities should consult their own tax advisors regarding the tax consequences of purchasing, owning and disposing of the ordinary shares or ADSs.
Passive Foreign Investment Company Considerations
A non-U.S. corporation, such as TCB, will be classified as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes, if, in the case of any particular taxable year, either (i) 75% or more of its gross income for such taxable year consists of certain types of “passive” income or (ii) 50% or more of the value of its assets (based on an average of the quarterly values of the assets) during such taxable year is attributable to assets that produce or are held for the production of passive income. For this purpose, cash is categorized as a passive asset and the company’s un-booked intangibles associated with active business activities may generally be classified as active assets. Passive income generally includes, among other things, dividends, interest, rents, royalties, and gains from the disposition of passive assets. For this purpose, a foreign corporation will be treated as owning its proportionate share of the assets and earning its proportionate share of the income of any other non-U.S. corporation in which it owns, directly or indirectly, more than 25% (by value) of the stock.
| -34- |
Based upon its current income and assets and projections as to the value of the ordinary shares or ADSs, it is not presently expected that we will be classified as a PFIC for the 2022 taxable year or the foreseeable future.
The determination of whether we will be or become a PFIC will depend upon the composition of its income (which may differ from our historical results and current projections) and assets and the value of its assets from time to time, including, in particular the value of its goodwill and other un-booked intangibles (which may depend upon the market value of the ordinary shares or ADSs from time to time and may be volatile). Among other matters, if our market capitalization is less than anticipated or subsequently declines, we may be classified as a PFIC for the taxable year in the 2021 taxable year or future taxable years. It is also possible that the IRS may challenge the classification or valuation of our assets, including its goodwill and other unbooked intangibles, or the classification of certain amounts received by us, including interest earnings, which may result in our being, or becoming classified as, a PFIC for the taxable year in 2021 or future taxable years.
The determination of whether we will be or become a PFIC may also depend, in part, on how, and how quickly, it uses liquid assets and the cash proceeds of this offering or otherwise. If we were to retain significant amounts of liquid assets, including cash, the risk of our being classified as a PFIC may substantially increase. Because there are uncertainties in the application of the relevant rules and PFIC status is a factual determination made annually after the close of each taxable year, there can be no assurance that we will not be a PFIC for the 2022 taxable year or any future taxable year, and no opinion of counsel has or will be provided regarding the classification of us as a PFIC. If we were classified as a PFIC for any year during which a holder held our ordinary shares or ADSs, it generally would continue to be treated as a PFIC for all succeeding years during which such holder held the ordinary shares or ADSs. The discussion below under “—Dividends Paid on Ordinary Shares or ADSs” and “—Sale or Other Disposition of Ordinary Shares or ADS” is written on the basis that we will not be classified as a PFIC for U.S. federal income tax purposes.
Dividends Paid on Ordinary Shares including ordinary shares represented by ADSs
Subject to the PFIC rules described below, any cash distributions (including constructive distributions) paid on the ordinary shares including ordinary shares represented by ADSs out of our current or accumulated earnings and profits, as determined under U.S. federal income tax principles, will generally be includible in the gross income of a U.S. Holder as dividend income on the day actually or constructively received by the U.S. Holder, in the case of ordinary shares including ordinary shares represented by ADSs. Because we do not intend to determine our earnings and profits on the basis of U.S. federal income tax principles, any distribution will generally be treated as a “dividend” for U.S. federal income tax purposes. Under current law, a non-corporate recipient of a dividend from a “qualified foreign corporation” will generally be subject to tax on the dividend income at the lower applicable net capital gains rate rather than the marginal tax rates generally applicable to ordinary income provided that certain holding period and other requirements are met.
A non-U.S. corporation (other than a corporation that is classified as a PFIC for the taxable year in which the dividend is paid or the preceding taxable year) will generally be considered to be a qualified foreign corporation (i) if it is eligible for the benefits of a comprehensive tax treaty with the United States which the Secretary of Treasury of the United States determines is satisfactory for these purposes and which includes an exchange of information program, or (ii) with respect to any dividend paid by such corporation on its stock, if such stock is readily tradable on an established securities market in the United States. We believe we are eligible for the benefits of the Convention Between the Government of the United States of America and the Government of the United Kingdom of Great Britain and Northern Ireland for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income and On Capital Gains, or the United States-United Kingdom income tax treaty (which the Secretary of the Treasury of the United States has determined is satisfactory for this purpose and includes an exchange of information program), in which case it would be treated as a qualified foreign corporation with respect to dividends paid on the ordinary shares or ADSs. U.S. Holders are urged to consult their tax advisors regarding the availability of the reduced tax rate on dividends in their particular circumstances. Dividends received on the ordinary shares will not be eligible for the dividends received deduction allowed to corporations.
| -35- |
Sale or Other Disposition of Ordinary Shares or ADSs
Subject to the PFIC rules discussed below, a U.S. Holder of our ordinary shares or ADSs will generally recognize capital gain or loss, if any, upon the sale or other disposition of ordinary shares or ADSs, respectively, in an amount equal to the difference between the amount realized upon the disposition and the U.S. Holder’s adjusted tax basis in such ordinary shares or ADSs. Any capital gain or loss will be long-term capital gain or loss if the ordinary shares or ADSs have been held for more than one year and will generally be United States source capital gain or loss for United States foreign tax credit purposes. Long-term capital gains of non-corporate taxpayers are currently eligible for reduced rates of taxation.
Disposition of Foreign Currency
U.S. Holders are urged to consult their tax advisors regarding the tax consequences of receiving, converting or disposing of any non-U.S. currency received as dividends on our ordinary shares or ADSs.
Tax on Net Investment Income
A U.S. Holder may be subject to a Medicare surtax of 3.8% on some or all of such U.S. Holder’s “net investment income” as defined in Section 1411 of the Code. Net investment income generally includes income from the ordinary shares or ADSs unless such income is derived in the ordinary course of the conduct of a trade or business (other than a trade or business that consists of certain passive or trading activities). You should consult your tax advisors regarding the effect this Medicare tax may have, if any, on your acquisition, ownership or disposition of ordinary shares or ADSs.
Passive Foreign Investment Company Rules
If we are is classified as a PFIC for any taxable year during which a U.S. Holder holds our ordinary shares or ADSs, unless the holder makes a mark-to-market election (as described below), the holder will, except as discussed below, be subject to special tax rules that have a penalizing effect, regardless of whether we remain a PFIC, on (i) any excess distribution that we make to the holder (which generally means any distribution paid during a taxable year to a holder that is greater than 125% of the average annual distributions paid in the three preceding taxable years or, if shorter, the holder’s holding period for the ordinary shares or ADSs), and (ii) any gain realized on the sale or other disposition, including, under certain circumstances, a pledge, of our ordinary shares or ADSs. Under the PFIC rules:
| ● | The excess distribution and/or gain will be allocated ratably over the U.S. Holder’s holding period for the ordinary shares or ADSs; | |
| ● | The amount of the excess distribution or gain allocated to the taxable year of the distribution or disposition and any taxable years in the U.S. Holder’s holding period prior to the first taxable year in which we are classified as a PFIC, or a pre-PFIC year, will be taxable as ordinary income; and | |
| ● | The amount of the excess distribution or gain allocated to each taxable year other than the taxable year of the distribution or disposition or a pre-PFIC year, will be subject to tax at the highest tax rate in effect applicable to the individuals or corporations, and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year. |
If we are a PFIC for any taxable year during which a U.S. Holder holds our ordinary shares or ADSs and any of its non-U.S. subsidiaries is also a PFIC, such holder would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC for purposes of the application of these rules. Each U.S. Holder is advised to consult its tax advisors regarding the application of the PFIC rules to any of our subsidiaries.
| -36- |
As an alternative to the foregoing rules, a U.S. Holder of “marketable stock” in a PFIC may make a mark-to-market election with respect to such ordinary shares or ADSs, provided that they are “regularly traded” (as specially defined under the Code) on The Nasdaq Stock Market. No assurances may be given regarding whether the ordinary shares or ADSs will qualify, or will continue to be qualified, as being regularly traded in this regard. If a mark-to-market election is made, the U.S. Holder will generally (i) include as ordinary income for each taxable year that we are a PFIC the excess, if any, of the fair market value of ordinary shares or ADSs held at the end of the taxable year over the adjusted tax basis of such securities and (ii) deduct as an ordinary loss the excess, if any, of the adjusted tax basis of such securities over the fair market value of such securities held at the end of the taxable year, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. The U.S. Holder’s adjusted tax basis in the ordinary shares or ADSs would be adjusted to reflect any income or loss resulting from the mark-to-market election. If a U.S. Holder makes an effective mark-to-market election, in each year that we are a PFIC any gain recognized upon the sale or other disposition of the ordinary shares or ADSs will be treated as ordinary income and loss will be treated as ordinary loss, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. U.S. Holders of our ordinary shares or ADSs should consult their tax advisors regarding the availability of a mark-to-market election with respect to such ordinary shares or ADSs.
If a U.S. Holder makes a mark-to-market election in respect of a corporation classified as a PFIC and such corporation ceases to be classified as a PFIC, the holder will not be required to take into account the mark-to-market gain or loss described above during any period that such corporation is not classified as a PFIC.
Because a mark-to-market election cannot be made for any lower-tier PFICs that a PFIC may own, a U.S. Holder who makes a mark-to-market election with respect to the ordinary shares or ADSs may continue to be subject to the general PFIC rules with respect to such holder’s indirect interest in any of our non-U.S. subsidiaries that is classified as a PFIC.
We do not intend to provide information necessary for U.S. Holder’s to make qualified electing fund elections, which, if available, would result in tax treatment different from the general tax treatment for PFICs described above. However, as described above under “Passive Foreign Investment Company Considerations-PFIC Classification of TCB,” it is not presently expected that we will be classified as a PFIC for the 2022 taxable year or the foreseeable future.
As discussed above under “Dividends Paid on Ordinary Shares or ADSs”, dividends that we pay on the ordinary shares or ADSs will not be eligible for the reduced tax rate that applies to qualified dividend income if we are classified as a PFIC for the taxable year in which the dividend is paid or the preceding taxable year. In addition, if a U.S. Holder owns the ordinary shares or ADSs during any taxable year that we are a PFIC, the holder must file an annual information return with the IRS. Each holder is urged to consult its tax advisor concerning the U.S. federal income tax consequences of purchasing, holding, and disposing ordinary shares or ADSs if we are or become a PFIC, including the possibility of making a mark-to-market election and the unavailability of the qualified electing fund election.
Information reporting and backup withholding
Certain U.S. Holders are required to report information to the IRS relating to an interest in “specified foreign financial assets,” including shares issued by a non-U.S. corporation, for any year in which the aggregate value of all specified foreign financial assets exceeds $50 thousand (or a higher U.S. dollar amount prescribed by the IRS), subject to certain exceptions (including an exception for shares held in custodial accounts maintained with a United States financial institution). These rules also impose penalties if a holder is required to submit such information to the IRS and fails to do so.
In addition, U.S. Holders may be subject to information reporting to the IRS and backup withholding with respect to dividends on and proceeds from the sale or other disposition of our ordinary shares or ADSs. Information reporting will apply to payments of dividends on, and to proceeds from the sale or other disposition of, our ordinary shares or ADSs by a paying agent within the United States to a holder, other than holders that are exempt from information reporting and properly certify their exemption. A paying agent within the United States will be required to withhold at the applicable statutory rate, currently 24%, in respect of any payments of dividends on, and the proceeds from the disposition of, our ordinary shares or ADSs within the U.S. to a U.S. Holder (other than holders that are exempt from backup withholding and properly certify their exemption) if the holder fails to furnish its correct taxpayer identification number or otherwise fails to comply with applicable backup withholding requirements. U.S. Holders who are required to establish their exempt status generally must provide a properly completed IRS Form W-9.
| -37- |
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a holder’s U.S. federal income tax liability. A U.S. Holder generally may obtain a refund of any amounts withheld under the backup withholding rules by filing the appropriate claim for refund with the IRS in a timely manner and furnishing any required information. Each U.S. Holder is advised to consult with its tax advisor regarding the application of the United States information reporting rules to their particular circumstances.
Material United Kingdom Tax Considerations
The following is a description of the material U.K. tax considerations relating primarily to the ownership and disposal of our ordinary shares or ADSs by the U.S. Holders described above. The U.K. tax comments set out below are based on current U.K. tax law as applied in Scotland, and HMRC practice (which may not be binding on HMRC) as at the date of this summary, both of which are subject to change, possibly with retrospective effect. They are intended as a general guide and, save where otherwise stated, only apply to you if you are not resident in the U.K. for U.K. tax purposes and do not hold our ordinary shares or ADSs for the purposes of a trade, profession or vocation that you carry on in the U.K. through a branch, agency or permanent establishment in the U.K. and if you hold our ordinary shares as an investment for U.K. tax purposes and are not subject to special rules.
This summary does not address all possible tax consequences relating to an investment in our ordinary shares or ADSs. In particular it does not cover the U.K. inheritance tax consequences of holding our ordinary shares or ADSs. It assumes that the depositary or DTC has not made an election under section 97A(1) of the Finance Act 1986. It assumes that we do not (and will not at any time) derive 75% or more of our qualifying asset value, directly or indirectly, from U.K. land, and that we are and remain solely resident in the U.K. for tax purposes. It assumes that the holder is not our officer or our employee (or of any related company of ours) and has not (and is not deemed to have) acquired the ordinary shares or ADSs by virtue of an office or employment. It assumes that a holder of ordinary shares or ADSs is the beneficial owner of the underlying ordinary shares for U.K. tax purposes. This summary is for general information only and is not intended to be, nor should it be considered to be, legal or tax advice to any particular holder. Holders of our ordinary shares or ADSs are strongly urged to consult their tax advisers in connection with the U.K. tax consequences of their investment in our securities.
U.K. Taxation of Dividends and Distributions
We will not be required to withhold amounts for or on account of U.K. tax at source when paying a dividend or distribution in respect of our ordinary shares.
Individual holders who hold our ordinary shares as an investment, who are not resident in the U.K. for U.K. tax purposes should not be subject to U.K. income tax in respect of any dividends on our ordinary shares, unless they hold their ordinary shares in connection with any trade, profession or vocation carried on (whether solely or in partnership) by them in the U.K. through a branch, agency or permanent establishment in the U.K.. In these circumstances, such holder may, depending on his or her individual circumstances, be chargeable to U.K. income tax in respect of our dividends.
Corporate holders which are not resident in the U.K. for U.K. tax purposes should not be subject to U.K. corporation tax in respect of any dividends on our ordinary shares, unless they carry on a trade in the U.K. through a permanent establishment to which the ordinary shares are attributable. In these circumstances, such holders may, depending on their individual circumstances and if an exemption from U.K. corporation tax in respect of dividend payments does not apply, be chargeable to U.K. corporation tax in respect of our dividends.
U.K. Taxation of Capital Gains
An individual holder who is not resident in the U.K. for U.K. tax purposes should not be liable to U.K. capital gains tax on capital gains realized on the disposal of their ordinary shares unless such holder carries on (whether solely or in partnership) a trade, profession or vocation in the U.K. through a branch or agency in the U.K. to which our ordinary shares are attributable. In these circumstances, such holder may, depending on his or her individual circumstances, be chargeable to U.K. capital gains tax on chargeable gains arising from a disposal of his or her ordinary shares.
| -38- |
Any such individual holder of our ordinary shares who is temporarily non-resident for U.K. tax purposes will, in certain circumstances, become liable to U.K. tax on capital gains in respect of gains realized while they were not resident in the U.K.
A corporate holder of our ordinary shares which is not resident in the U.K. for U.K. tax purposes should not be liable for U.K. corporation tax on chargeable gains realized on the disposal of our ordinary shares unless it carries on a trade in the U.K. through a permanent establishment in the U.K. to which our ordinary shares are attributable. In these circumstances, a disposal of ordinary shares by such holder may give rise to a chargeable gain or an allowable loss for the purposes of U.K. corporation tax.
Stamp Duty and Stamp Duty Reserve Tax
The discussion below relates to the holders of our ordinary shares or ADSs wherever resident, however it should be noted that special rules may apply to certain persons such as market makers, brokers, dealers or intermediaries.
As a general rule (and except in relation to depositary receipt systems and clearance services (as to which see below)), no UK stamp duty or stamp duty reserve tax, or SDRT, is payable on the issue of the ordinary shares underlying the ADSs.
An unconditional agreement to transfer ordinary shares will normally give rise to a charge to SDRT at the rate of 0.5% of the amount or value of the consideration payable for the transfer. The purchaser of the shares is liable for the SDRT. Transfers of ordinary shares in certificated form are generally also subject to stamp duty at the rate of 0.5% of the amount or value of the consideration given for the transfer (rounded up to the next £5.00). Stamp duty is normally paid by the purchaser. The charge to SDRT will be cancelled or, if already paid, repaid (generally with interest), where a transfer instrument has been duly stamped within six years of the charge arising, (either by paying the stamp duty or by claiming an appropriate relief) or if the instrument is otherwise exempt from stamp duty.
Under current UK legislation, an issue or transfer of ordinary shares or an unconditional agreement to transfer ordinary shares to a clearance service or a depositary receipt system (including to a nominee or agent for, a person whose business is or includes the issue of depositary receipts or the provision of clearance services) will generally be subject to SDRT (and, in the case of transfers, where the transfer is effected by a written instrument, stamp duty) at a higher rate of 1.5% of the amount or value of the consideration given for the transfer or, in certain circumstances, the value of the ordinary shares unless the clearance service has made and maintained an election under section 97A of the UK Finance Act 1986, or a section 97A election. It is understood that HMRC regards the facilities of DTC as a clearance service for these purposes and we are not aware of any section 97A election having been made by the DTC.
However, based on current published HMRC practice following European Union case law in respect of the European Council Directives 69/335/EEC and 2009/7/EC, no SDRT is generally payable in respect of such an issue of ordinary shares and no SDRT or stamp duty is generally payable in respect of such a transfer of ordinary shares where such transfer is an integral part of an issue of share capital. It is noted that on January 31, 2020 the United Kingdom ceased to be a Member State of the European Union. Accordingly, the extent to which HMRC’s position will remain as set out in this paragraph following the end of the transition period on December 31, 2020 is uncertain.
Any stamp duty or SDRT payable on an issue or transfer of ordinary shares to a depositary receipt system or clearance service (although strictly accountable by the clearance service or depositary receipt system operator or their nominee) will in practice generally be paid by the transferors or participants in the clearance service or depositary receipt system. Specific professional advice should be sought before incurring or reimbursing the costs of a 1.5% stamp duty or SDRT charge in any circumstances.
No UK SDRT or stamp duty is required to be paid in respect of the issue or transfer of, or an agreement to transfer, ADSs (including by way of a paperless transfer of ADSs through the facilities of DTC).
| -39- |
The ADSs being offered by the selling shareholder are those issuable to the selling shareholder upon exercise of the Warrants. For additional information regarding the issuances of those securities, see “Recent Developments – August 2023 Warrant Inducement” above. We are registering the ADSs in order to permit the selling shareholders to offer the ADSs issuable upon exercise of the Warrants for resale from time to time. Except for the ownership of the Company’s securities, the selling shareholder has not had any material relationship with us within the past three years.
The table below lists the selling shareholder and other information regarding the beneficial ownership of the ADSs by the selling shareholder. The second column lists the number of ADSs beneficially owned by the selling shareholder, based on its ownership of the ADSs and warrants, as of June 19, 2024, assuming exercise of the Warrants held by the selling shareholders on that date, without regard to any limitations on exercises.
The third column lists the ADSs being offered by this prospectus by the selling shareholder.
In accordance with the terms of a warrant inducement agreement with the selling shareholder, this prospectus generally covers the resale of the number of ADSs issuable upon exercise of the Warrants issued to the selling shareholders in the “Recent Developments – May 2024 Warrant Inducement” described above, determined as if the outstanding Warrants were exercised in full as of the trading day immediately preceding the date this registration statement was initially filed with the SEC. The fourth column assumes the sale of all of the ADSs offered by the selling shareholder pursuant to this prospectus.
Under the terms of the Warrants, a selling shareholder may not exercise any such warrants to the extent such exercise would cause such selling shareholder, together with its affiliates and attribution parties, to beneficially own a number of ADSs which would exceed 4.99% of our then outstanding ADSs following such exercise, excluding for purposes of such determination ADSs issuable upon exercise of such warrants which have not been exercised. The number of ADSs in the second and fourth columns do not reflect this limitation. The selling shareholders may sell all, some or none of their shares in this offering. See “Plan of Distribution.”
| Name of Selling Shareholder | Number of ADSs Owned Prior to Offering | Maximum Number of ADSs to be Sold Pursuant to this Prospectus | Number of ADSs Owned After Offering | |||||||||
| Armistice Capital, LLC (1) | 3,501,882 | 3,500,000 | 1,882 | |||||||||
(1) Includes (i) 3,500,000 ADSs issuable upon exercise of Warrants, and (ii) 1,882 ADSs issuable upon exercise of publicly traded warrants. The securities are directly held by Armistice Capital Master Fund Ltd., a Cayman Islands exempted company (the “Master Fund”), and may be deemed to be indirectly beneficially owned by: (i) Armistice Capital, LLC, or Armistice Capital, as the investment manager of the Master Fund; and (ii) Steven Boyd, as the Managing Member of Armistice Capital. Armistice Capital and Steven Boyd disclaim beneficial ownership of the securities except to the extent of their respective pecuniary interests therein. Of the total number of shares identified in the column entitled ‘Maximum Number of ADSs to be Sold Pursuant to this Prospectus’ above, such ADSs are subject to a beneficial ownership limitation preventing the Master Fund from exercising any portion of the Warrants if such exercise would result in the Master Fund owning greater than 4.99% of our outstanding shares following such exercise. The address of the Master Fund is c/o Armistice Capital, LLC, 510 Madison Ave, 7th Floor, New York, NY 10022.
| -40- |
The selling shareholder and any of its pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their Ordinary Shares represented by ADSs covered by this prospectus on the principal Trading Market or any other stock exchange, market or trading facility on which the securities are traded or in private transactions. These sales may be at fixed or negotiated prices. A selling shareholders may use any one or more of the following methods when selling securities:
| ● | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; | |
| ● | block trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction; | |
| ● | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; | |
| ● | an exchange distribution in accordance with the rules of the applicable exchange; | |
| ● | privately negotiated transactions; | |
| ● | settlement of short sales; | |
| ● | in transactions through broker-dealers that agree with the selling shareholders to sell a specified number of such securities at a stipulated price per security; | |
| ● | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; | |
| ● | a combination of any such methods of sale; or | |
| ● | any other method permitted pursuant to applicable law. |
The selling shareholder may also sell securities under Rule 144 or any other exemption from registration under the Securities Act of 1933, as amended (the “Securities Act”), if available, rather than under this prospectus.
Broker-dealers engaged by the selling shareholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling shareholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this Prospectus, in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or markdown in compliance with FINRA Rule 2121.
In connection with the sale of the securities or interests therein, the selling shareholder may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they assume. The selling shareholders may also sell securities short and deliver these securities to close out their short positions, or loan or pledge the securities to broker-dealers that in turn may sell these securities. The selling shareholder may also enter into option or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The selling shareholder and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. The selling shareholder has informed the Company that it does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the securities.
The Company is required to pay certain fees and expenses incurred by the Company incident to the registration of the securities. The Company has agreed to indemnify the selling shareholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
We agreed to keep this prospectus effective until all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
| -41- |
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously engage in market making activities with respect to the ADSs for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the selling shareholders will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the ADSs by the selling shareholders or any other person. We will make copies of this prospectus available to the selling shareholders and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under the Securities Act).
Set forth below is an itemization of the total anticipated expenses, excluding underwriting discounts, expected to be incurred in connection with the offer and sale of the ADSs by us. With the exception of the SEC registration fee and the FINRA filing fee, all amounts are estimates, in United States dollars:
| SEC registration fee | $ | 584 | ||
| Printer fees and expenses | $ | - | ||
| Legal fees and expenses | $ | 25,000 | ||
| Accounting fees and expenses | $ | 20,000 | ||
| Miscellaneous | $ | 2,500 | ||
| Total | $ | 48,084 |
We are being represented by Sheppard, Mullin, Richter & Hampton LLP, New York, New York with respect to certain legal matters of United States federal securities and New York state law. We are being represented by Addleshaw Goddard LLP, Glasgow, Scotland with respect to certain legal matters of the law of Scotland and other applicable law of the United Kingdom and as to certain patent law matters by Murgitroyd & Company Limited. The validity of the ordinary shares offered in this offering and legal matters as to the law of Scotland were passed upon for us by Addleshaw Goddard LLP, Glasgow, Scotland.
The consolidated financial statements of TC BioPharm (Holdings) plc incorporated by reference in TC BioPharm (Holdings) plc’s Annual Report (Form 10-K) for the years ended December 31, 2023 and December 31, 2022, have been audited by Marcum LLP, independent registered public accounting firm, as set forth in their report thereon, (which contains an explanatory paragraph describing conditions that raise substantial doubt about the Company’s ability to continue as a going concern as described in Note 1 to the consolidated financial statements) included therein, and incorporated herein by reference. Such consolidated financial statements are incorporated herein by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The registered business address of Marcum LLP is 730 3rd Avenue, 11th Floor, New York, NY 10017, United States of America.
| -42- |
WHERE YOU CAN FIND MORE INFORMATION
We are a reporting company and file annual, quarterly and current reports, proxy statements and other information with the SEC. We have filed with the SEC a registration statement on Form S-3 under the Securities Act with respect to the sale, from time to time, of the shares of Common Stock held by the selling stockholders named in this prospectus and any applicable prospectus supplement.
This prospectus does not contain all of the information set forth in the registration statement and the exhibits to the registration statement. For further information with respect to us and the securities being offered under this prospectus, we refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement.
You may read and copy the registration statement, as well as our reports, proxy statements and other information, on the SEC’s website at http://www.sec.gov. You can also obtain copies of materials we file with the SEC from our website found at www.tcbiopharm.com. Information on our website does not constitute a part of, nor is it incorporated in any way, into this prospectus and should not be relied upon in connection with making an investment decision.
INCORPORATION OF DOCUMENTS BY REFERENCE
The SEC allows us to “incorporate by reference” information into this prospectus, which means that we can disclose important information to you by referring you to another document filed separately with the SEC. The documents incorporated by reference into this prospectus contain important information that you should read about us.
The following documents are incorporated by reference into this prospectus and any applicable prospectus supplement:
| ● | our Annual Report on Form 10-K/A for the fiscal year ended December 31, 2023, filed with the SEC on April 29, 2024; | |
| ● | our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on April 1, 2024; | |
| ● | our Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2024, filed with the SEC on May 15, 2024; | |
| ● | our Current Reports on Form 8-K (other than Current Reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items) filed with the SEC on January 4, 2024, February 14, 2024, March 6, 2024, March 12, 2024, March 18, 2024, March 19, 2024, April 4, 2024, May 6, 2024, May 8, 2024, May 20, 2024 and May 29, 2024; | |
| ● | our definitive Proxy Statement on Schedule 14A for our 2024 Annual Meeting of Shareholders, filed with the SEC on June 7, 2024; | |
| ● | the description of our Common Stock contained in our registration statement on Form 8-A (File No. 001-41231) filed with the SEC on January 14, 2022, including any amendments or reports filed with the SEC for the purposes of updating such description. |
All documents subsequently filed by us (other than Current Reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items unless such Form 8-K expressly provides to the contrary) with the SEC pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act, including those made after the date of the initial filing of the registration statement of which this prospectus forms a part and prior to effectiveness of such registration statement, until we file a post-effective amendment that indicates the termination of the offering of the shares of Common Stock made by this prospectus are deemed to be incorporated by reference into this prospectus. Such future filings will become a part of this prospectus from the respective dates that such documents are filed with the SEC.
Any statement contained herein or in a document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified or superseded for purposes hereof to the extent that such statement contained herein or in any other subsequently filed document, which is also incorporated or deemed to be incorporated herein, modifies or supersedes such statement. Any such statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
You can obtain any of the filings incorporated by reference into this prospectus through us or from the SEC through the SEC’s website at http://www.sec.gov. We will provide, without charge, to each person, including any beneficial owner, to whom a copy of this prospectus is delivered, upon written or oral request of such person, a copy of any or all of the reports and documents referred to above which have been or may be incorporated by reference into this prospectus. You should direct requests for those documents to:
TC BioPharm (Holdings) plc
Maxim 1, 2 Parklands Way
Holytown, Motherwell, ML1 4WR
Scotland, United Kingdom
+44 (0) 141 433 7557
We maintain an internet site at http://www.tcbiopharm.com. Our website and the information contained on or connected to it shall not be deemed to be incorporated into this prospectus or the registration statement of which it forms a part.
| -43- |

UP TO 3,500,000
AMERICAN DEPOSITARY SHARES
REPRESENTING 3,500,000 ORDINARY SHARES
TC BIOPHARM (HOLDINGS) PLC
PROSPECTUS
June 24, 2024