TC BioPharm Announces Positive Phase 1b/2a Data in Late-Stage Acute Myeloid Leukemia Patients Treated with Allogeneic Gamma Delta T Cells
-Complete and near complete responses (morphologic leukemia-free state) observed with OmnImmune® starting at lowest dose level in Phase 1b/2a study for the treatment of relapsed/refractory acute myeloid leukemia
-Results yielded no related serious adverse events, including no Graft v Host Disease, neurotoxicity or cytokine release syndrome at any dose level
Allogeneic product can be stored frozen and used as an 'off-the-shelf' cell therapy
EDINBURGH, Scotland, March 8, 2022 /PRNewswire/ -- TC Biopharm (Holdings) PLC ("TC Biopharm" or the "Company") (NASDAQ: TCBP) (NASDAQ: TCBPW), a clinical stage biotechnology company developing platform allogeneic gamma-delta T cell therapies for cancer and viral indications, announces positive interim data its Phase 1a/2b human study evaluating safety and tolerability of TCB-002, OmnImmune®, the Company's allogeneic unmodified gamma delta t-cell product, a novel therapeutic targeting the potential treatment of relapse/refractory Acute Myeloid Leukemia ("AML").
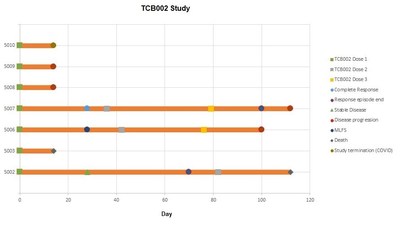
7 patients received treatment and 2 patients (enrolled in the low dose cohort) did not reach day 28 assessment and were not evaluable for efficacy per protocol with the third patient being available for safety inclusion but not efficacy.
Of the 7 patients treated, three received OmnImmune®, at a low-dose and four received OmnImmune®, at a higher dose of cells. In the low dose cohort one patient achieved MLFS (morphologic leukemia-free state), one patient achieved stable disease characterized as near complete response and one patient met safety endpoints but was lost to follow-up due to disease co-morbidity (bilateral pneumonia unrelated to OmnImmune® treatment). In the higher dose cohort, 50% of patients achieved complete responses, one patient had progressive disease and one patient exhibited significantly reduced cancer blast count at day fourteen (prior to the study being cut short due to Covid 19).
"We are extremely pleased to receive such positive data from our phase 1b/2a study demonstrating OmnImmune® as safe and tolerable among patients with advanced acute Myeloid Leukemia," said Bryan Kobel, Chief Executive Officer of TC BioPharm. "These results underline OnmImmune's potential in becoming a viable AML treatment. We look forward to reporting additional phase 2/3 clinical data during the first half of 2022."
"These results are very encouraging in such late-stage unresponsive patients, most of whom had very advanced cancer, were fourth or fifth line with no further therapeutic options," said Dr. Sebastian Wanless, Senior Clinical Director at TC BioPharm. "OmnImmune® comprises allogeneic gamma delta T cells sourced from healthy donors, expanded and activated in large numbers before being purified and formulated for infusion into patients. Our Phase 2b/3 trial will take this therapeutic another step further, incorporating our frozen/thawed product generated from our proprietary universal cell banks of gamma deltas, for a true off the shelf allogeneic cell therapy. During 2022 we plan to evaluate OmnImmune® efficacy in unresponsive first-line of treatment AML patients and expand our clinical trial efforts into multiple blood cancer indications."
Allogeneic gamma-delta T cell persistence was evaluated in two patients treated. In one patient allogenic product remained detectable after 100 days following three infusions. Another patient demonstrated haematological recovery with sustained elevation in key immune cells over 100 days following the initial infusion.
Clinical data provided evidence of good safety and tolerability profile of OmnImmune® as no product related safety concerns were raised during Safety Review Committee meetings. Moreover, no graft vs host disease, immune effector cell-associated neurotoxicity syndrome or cytokine release syndrome was reported in any of the treated patients.
The two patients exhibiting complete response and stable disease were re-dosed with allogeneic product, and the patient with and morphologic leukemia-free state received two further infusions of OmnImmune®. No toxicities were documented following these repeat infusions.
Dr. Michael Leek, Founder and Executive Chairman commented, "Apart from matched bone-marrow stem cell transplants, patients with unresponsive AML are presented with few viable treatment options. At TC BioPharm we are developing affordable allogeneic cell therapies for blood cancers such as AML, we believe such cell therapies will become a future mainstream standard of care for hematological malignancies."
About OmnImmune®
OmnImmune® an allogeneic unmodified cell therapy consisting of activated and expanded gamma delta T cells. The trial, for treatment of patients suffering from relapse/refractory Acute Myeloid Leukemia (AML), OmnImmune® comprises GDT cells sourced from healthy donors, expanded and activated in large numbers before being purified and formulated for infusion into patients. OmnImmune® is a frozen and thawed product, now "banked" from donor derived cells. Phase 2/3 trials will begin enrollment in the first quarter of 2022 in the UK with expansion into the US in 2022.
About TC BioPharm, plc.
TC BioPharm is a clinical-stage biopharmaceutical company focused on the discovery, development and commercialization of gamma-delta T cell therapies for the treatment of cancer and viral infections with human efficacy data in acute myeloid leukemia. Gamma-delta T cells are naturally occurring immune cells that embody properties of both the innate and adaptive immune systems and can intrinsically differentiate between healthy and diseased tissue. TC BioPharm uses an allogeneic approach in both unmodified and CAR modified gamma delta t-cells to effectively identify, target and eradicate both liquid and solid tumors in cancer.
TC BioPharm is the leader in developing gamma-delta T cell therapies, and the first company to conduct phase II/pivotal clinical studies in oncology. The Company is conducting two investigator-initiated clinical trials for its unmodified gamma-delta T cell product line - Phase 2b/3 pivotal trial for OmnImmune in treatment of acute myeloid leukemia and Phase I trial for ImmuniStim in treatment of Covid patients using the Company's proprietary allogenic CryoTC technology to provide frozen product to clinics worldwide. TC BioPharm also maintains a robust pipeline for future indications in solid tumors and other aggressive viral infections as well as a significant IP/patent portfolio in the use of CARs with gamma delta t-cells and owns our manufacturing facility to maintain cost and product quality controls.
Forward Looking Statements
This press release may contain statements of a forward-looking nature relating to future events. These forward-looking statements are subject to the inherent uncertainties in predicting future results and conditions. These statements reflect our current beliefs, and a number of important factors could cause actual results to differ materially from those expressed in this press release. We undertake no obligation to revise or update any forward-looking statements, whether as a result of new information, future events or otherwise.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/tc-biopharm-announces-positive-phase-1b2a-data-in-late-stage-acute-myeloid-leukemia-patients-treated-with-allogeneic-gamma-delta-t-cells-301496932.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/tc-biopharm-announces-positive-phase-1b2a-data-in-late-stage-acute-myeloid-leukemia-patients-treated-with-allogeneic-gamma-delta-t-cells-301496932.html
SOURCE TC BioPharm
Released March 8, 2022